Figures & data
Figure 1 LATTE-2 study design. IM, intramuscular; PO, oral; Q4W, every 4 weeks; Q8W, every 8 weeks; QD, once daily. aParticipants who withdrew after at least 1 IM dose entered the long-term follow-up period.
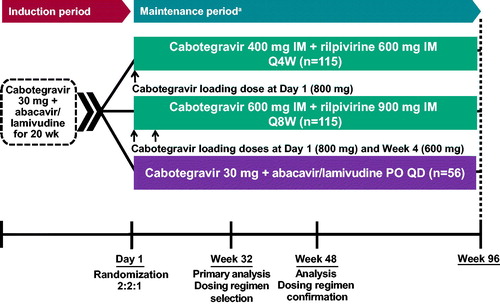
Table 1 Item content in the HIVTSQ[s] and HIVTSQ[c] versions.Citation11,a
Figure 2 Participants reporting ISRs by visit through Week 96 of the maintenance period. AE, adverse event; IM, intramuscular; ISR, injection-site reaction; Q4W, every 4 weeks; Q8W, every 8 weeks. In the Q8W group, one participant completed the maintenance phase but did not receive injections at Week 96.
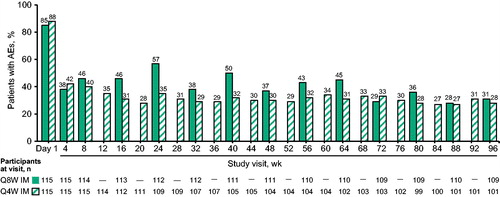
Table 2 Injection-site reactions in the cabotegravir + rilpivirine long-acting injectable treatment groups during the maintenance period through week 96.
Figure 3 Amount of pain/discomfort with cabotegravir LA + rilpivirine LA versus oral cabotegravir plus abacavir/lamivudine at Week 8 and Week 96 (HIVMQ, item F). Unlabeled bar values corresponding to item scores 3–6 represent ≤8% of respondents per bar. HIVMQ, HIV Medication Questionnaire; LA, long acting.
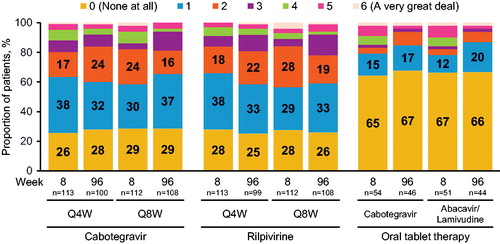
Table 3 Change over time in patient-reported inconvenience associated with cabotegravir and rilpivirine injections and oral tablet therapy (HIVMQ).
Figure 4 (A) HIVTSQ[s] total scores at Day 1, Weeks 8, and 96. Boxplot values represent the median (center) and first and third quartiles (bottom and top); error bars represent the minimum and maximum scores. Comparisons made using the Wilcoxon rank sum test. Q4W, n = 100; Q8W, n = 108; PO, n = 46. (B) Response scores by item label on the general satisfaction/clinical subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded and grouped from the questionnaire’s seven-point scale as a post hoc analysis: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. (C) Response scores by item label on the lifestyle/ease subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded from questionnaire’s seven-point scale: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. HIVTSQ[c], HIV Treatment Satisfaction Questionnaire, change version; HIVTSQ[s], HIV Treatment Satisfaction Questionnaire, status version; LA: long acting; PO: once-daily oral tablets; SD: standard deviation; Q4W: cabotegravir LA + rilpivirine LA once every 4 weeks; Q8W: cabotegravir LA + rilpivirine LA once every 8 weeks. *P = 0.02 (post hoc) compared with oral dosing at Week 96. **P < 0.001 (post hoc) compared with oral dosing at Week 96. aItems added to the standard HIVTSQ specifically for the LATTE-2 study.
![Figure 4 (A) HIVTSQ[s] total scores at Day 1, Weeks 8, and 96. Boxplot values represent the median (center) and first and third quartiles (bottom and top); error bars represent the minimum and maximum scores. Comparisons made using the Wilcoxon rank sum test. Q4W, n = 100; Q8W, n = 108; PO, n = 46. (B) Response scores by item label on the general satisfaction/clinical subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded and grouped from the questionnaire’s seven-point scale as a post hoc analysis: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. (C) Response scores by item label on the lifestyle/ease subscale of the HIVTSQ[c] for participants on LA injectable therapy at Week 32 compared with oral treatment prior to randomization. Item responses were recoded from questionnaire’s seven-point scale: less favorable versus induction treatment, −3 to −1; neutral versus induction treatment, 0; more favorable versus induction treatment, 1–3. Unlabeled bar values correspond to ≤4% of respondents per bar. Q4W, n = 100; Q8W, n = 107; PO, n = 49. HIVTSQ[c], HIV Treatment Satisfaction Questionnaire, change version; HIVTSQ[s], HIV Treatment Satisfaction Questionnaire, status version; LA: long acting; PO: once-daily oral tablets; SD: standard deviation; Q4W: cabotegravir LA + rilpivirine LA once every 4 weeks; Q8W: cabotegravir LA + rilpivirine LA once every 8 weeks. *P = 0.02 (post hoc) compared with oral dosing at Week 96. **P < 0.001 (post hoc) compared with oral dosing at Week 96. aItems added to the standard HIVTSQ specifically for the LATTE-2 study.](/cms/asset/f48cac80-8fc2-411e-991c-eefa938dc0a8/yhct_a_1661696_f0004_c.jpg)
Data availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
