Figures & data
Table 1. Discontinuations of DTG or other INSTIs, and reasons for discontinuation.
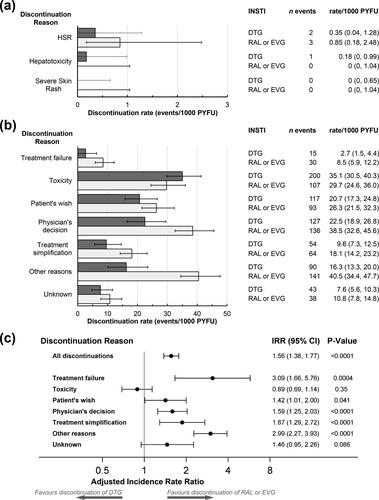
Figure 1. Discontinuations of cART regimens with DTG (649 discontinuations during 5649 PYFU) or with other integrase inhibitors (RAL or EVG, 612 discontinuations during 3531 PYFU). Rates of discontinuations (with 95% confidence intervals) for independently reviewed hypersensitivity reaction (HSR), hepatotoxicity or severe skin rash events (a) or for other discontinuation reasons (b). Numbers of discontinuations and discontinuation incidence rates are shown on the right. Discontinuation rates were similar for RAL or EVG, except that of 30 discontinuations for treatment failure with RAL or EVG, 12 were for RAL (discontinuation rate 7.4 (95% CI 4.2–13.0)/1000 PYFU) and 18 for EVG (discontinuation rate 9.5 (6.0–15.0)/1000 PYFU). Similarly of 107 discontinuations for toxicity, 39 were for RAL (discontinuation rate 23.9 (95% CI 17.5–32.7)/1000 PYFU) and 68 for EVG (discontinuation rate 35.8 (28.2–45.4)/1000 PYFU), and of 64 discontinuations for treatment simplification, 57 were for RAL (discontinuation rate 34.9 (95% CI 27.0–45.3)/1000 PYFU) and 7 for EVG (discontinuation rate 3.7 (1.8–7.7)/1000 PYFU). (c) Adjusted discontinuations incidence rate ratios (IRRs) comparing discontinuations of RAL or EVG-containing regimens with DTG discontinuations (reference). Models were adjusted for participant’s characteristics at baseline (sex, ethnicity, risk group, region, ART naïve or INSTI naïve, prior use of PIs, NNRTIs or DTG, EVG or RAL, the total number of ARVs previously exposed to, and time since first ARV use) or time-updated variables (age, body mass index, smoking status, AIDS or non-AIDS clinical conditions, diabetes, hypertension, anemia, hepatitis B or C virus co-infection, as well as current and peak viral load, current and nadir CD4 counts, estimated glomerular filtration rate, ALT and AST levels and the proportion of follow-up time with low CD4 counts or high viral load). Factors that were significant in univariate analyses (p < 0.1) were included in multivariate models; excluded variables were added in turn to determine if their inclusion improved the fit of the model (Note: Due to low numbers of events, the aIRR for treatment failure was only adjusted for sex, CD4 cell counts at baseline and ARV treatment naïve). Abbreviations: ALT, alanine aminotransferase; ART, anti-retroviral therapy; AST, aspartate aminotransferase; DTG, dolutegravir; EVG, elvitegravir; HSR, hypersensitivity reaction; INSTI, integrase strand transfer inhibitor; IRR, incidence rate ratio; NNRTI, non- nucleotide reverse-transcriptase inhibitor; PI, protease inhibitor; PYFU, person-years of follow-up; RAL, raltegravir.