Figures & data
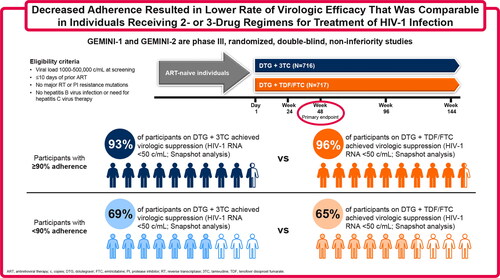
Table 1. Baseline characteristics by adherence category in GEMINI-1 and GEMINI-2 (ITT-E population).
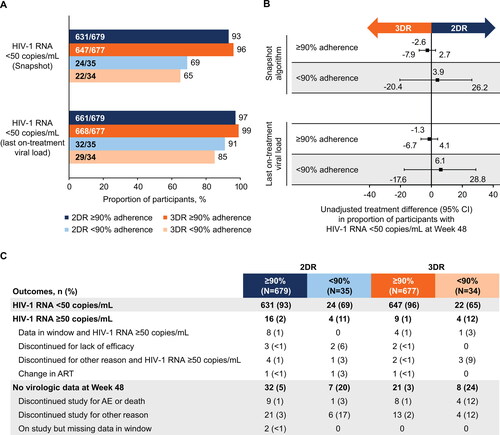
Figure 1. (A) Proportion of participants with HIV-1 RNA <50 copies/mL at Week 48 using the FDA Snapshot algorithm and last on-treatment viral load by adherence category, (B) unadjusted treatment differences (95% CI) between groups, and (C) Snapshot outcomes by adherence category. AE, adverse event; ART, antiretroviral therapy; 2DR, dolutegravir + lamivudine; 3DR, dolutegravir + tenofovir disoproxil fumarate/emtricitabine; FDA, US Food and Drug Administration.
Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
