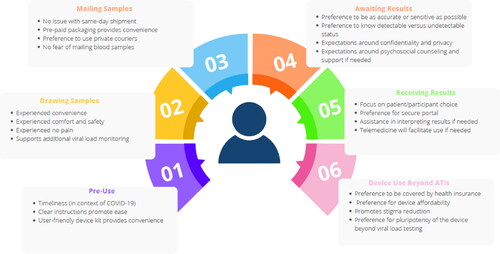
Figures & data
Figure 1 Experimental blood collection device for home-based viral load testing under development by Tasso, Inc. and Merck & Co, Inc., Kenilworth, NJ, USA.
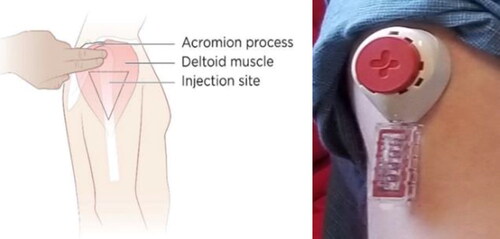
Table 1 Demographic characteristics of study participants (Philadelphia, PA, United States, 2021–2022).
yhct_a_2103582_sm0750.zip
Download Zip (112.2 KB)Data availability
All data relevant to this study have been provided in the text and in the Supplementary Appendices.