Figures & data
Figure 1. Immunity against Mycobacterium tuberculosis (MTb) and the effects of HIV. (A) Alveolar macrophages (AMs) are the first cells to encounter and engulf MTb bacteria when they are inhaled deeply into the lungs. MTb bacteria have evolved to escape intracellular killing by AMs by arresting phagosomal maturation and possibly escaping the phagosome to allow for persistence and growth within AMs. The defense mechanisms against this include chemokines/cytokine secretion which activates antimycobacterial defenses and adaptive immunity, autophagy (59), and apoptosis (51) among others. (B) HIV is known to affect a number of these steps, including increased phagocytosis of MTb to allow access to intracellular environment, decreased AM apoptosis in response to MTb, decreased autophagy, and decreased chemokine/cytokine production. HIV also affects function and numbers of CD41 T cells, leading to increased bacillary loads, inadequate granuloma formation, and dissemination. DC 5 dendritic cell; TNF 5 tumor necrosis factor.
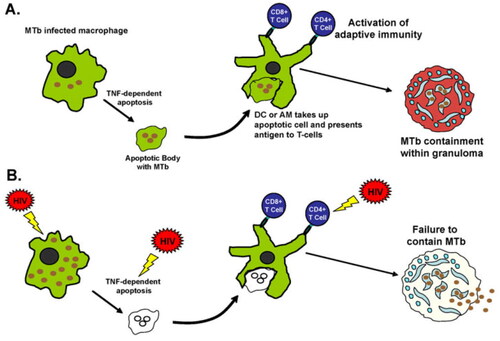
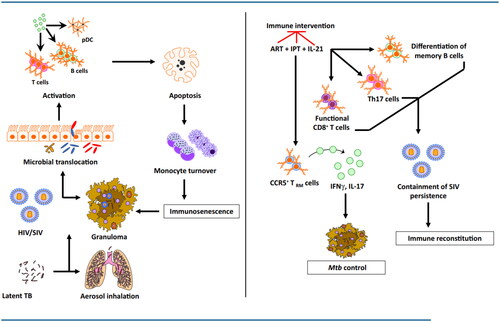
Figure 2. HIV co-infection with Mtb is characterized by immune activation encompassing a wide array of tissues and cells. HIV co-infection leads to a drastic depletion of CD4+ T cells by loss of mucosal integrity and, in turn, a loss of immune function in the gastrointestinal tract. This causes a translocation of resident microbial products into the systemic circulation leading to the activation of several cell types, including T, B, and NK cells, plasmacytoid dendritic cells (pDCs), and monocytes. In addition to producing proinflammatory cytokines, these activated cell subsets demonstrate increased apoptosis and turnover. The integrity of the granuloma structure in a reactivated macaque is maintained by this increased monocyte turnover that replaces the apoptotic macrophages. The HIV infection promotes macrophage killing, leading to the breakdown of granulomas, which in turn leads to a breach of Mtb containment and reactivation. While antiretroviral therapy (ART) successfully contains the virus, it fails to resolve the chronic immune activation completely. Concurrent therapy with isoniazid and/or IL-21 could achieve both bacterial containment and immune activation. While isoniazid treatment in conjunction with ART could restore CCR5+ TRM cells in the lung tissues leading to better control of Mtb replication in the macrophages, IL-21 could serve to promote the maintenance and functionality of Th17 cells, B cells, and CD8+ T cells. Together, this novel therapy could potentially lead to better immune reconstitution and resolve virus-driven residual immune activation in a Mtb/HIV co-infection.