Figures & data
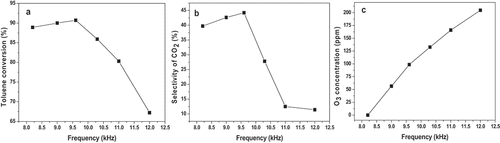
Figure 1. Schematic diagram of the experimental set-up. 1- Air cylinder 2- Mass flow meter 3- Ice-water mixture bath 4- Gas mixer 5- Oscilloscope 6- Plasma reactor (discharge zone) 7- Catalyst 8- Gas chromatography 9- Ozone analyzer.
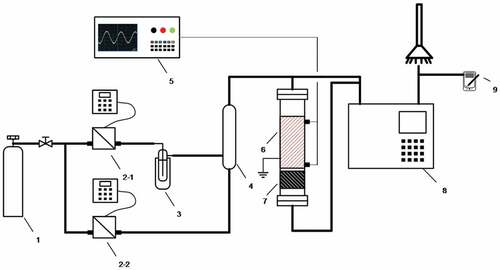
Figure 2. a). Effect of discharge length on toluene conversion; b). Effect of discharge length on CO2 selectivity; c). Effect of discharge length on ozone generation.
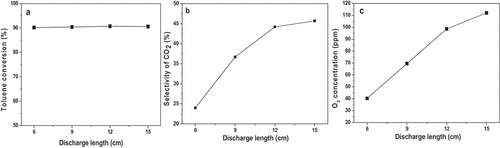
Figure 3. a). Effect of discharge voltage on toluene conversion; b). Effect of discharge voltage on CO2 selectivity; c). Effect of discharge voltage on ozone generation.
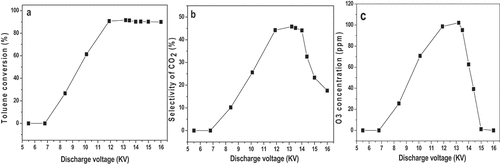
Figure 4. a). Effect of frequency on toluene conversion; b). Effect of frequency on CO2 selectivity; c). Effect of frequency on ozone generation.

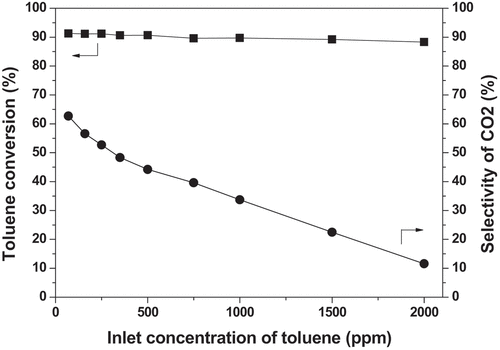
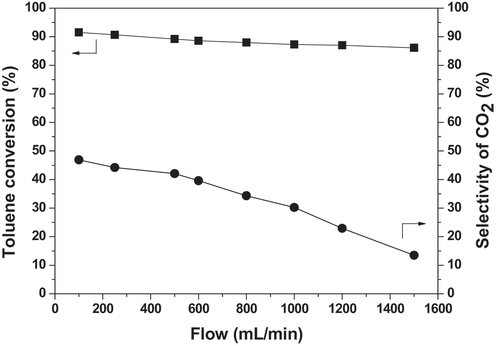
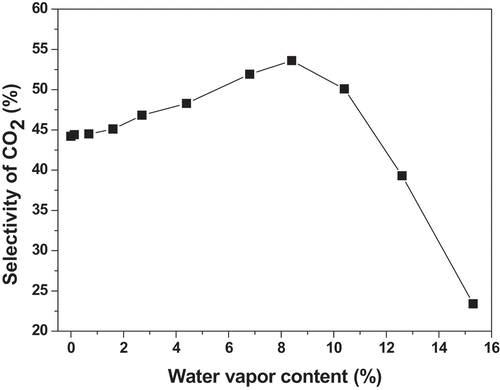
![Figure 8. Performances of toluene removal in the plasma alone and plasma-catalysis system (Reaction conditions: Room temperature; Atmosphere pressure; [C7H8 concentration] = 70 ppm, [C7H8 flow rate] = 1 L/min, GHSV = 18,000 h−1).](/cms/asset/70a89c37-6813-4168-a81b-f138187131b5/tcsb_a_2065363_f0008_oc.jpg)
![Figure 9. Outlet ozone concentration as a function of time in the plasma alone and plasma-catalysis system (Reaction conditions: Room temperature; Atmosphere pressure and [C7H8 concentration] = 70 ppm, [C7H8 flow rate] = 1 L/min and GHSV = 18,000 h−1).](/cms/asset/62923364-de73-4ea6-8b2b-9de149b8036f/tcsb_a_2065363_f0009_oc.jpg)