Figures & data
Table 1. Elemental contents of p-BC, C2H6O-BC and C2H6O-BC after nor adsorption.
Figure 1. SEM images of p-BC (a) and C2H6O-BC (b), N2 adsorption and desorption isotherms (c) and FT-IR spectra of unloaded and NOR/Cu-loaded biochars (d).
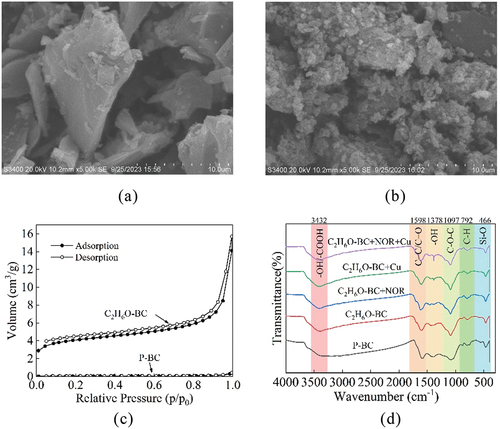
Figure 2. Adsorption isotherms, kinetics and the related comparisons and fitting [(a) adsorption isotherms; (b) comparison of NOR adsorption capacity by C2H6O-BC with that by unmodified/modified hydrothermal/slow pyrolyzed biochars reported in literature [Citation34–38]; (c) adsorption kinetics ; and (d) mass transfer process simulated by weber-morris model].
![Figure 2. Adsorption isotherms, kinetics and the related comparisons and fitting [(a) adsorption isotherms; (b) comparison of NOR adsorption capacity by C2H6O-BC with that by unmodified/modified hydrothermal/slow pyrolyzed biochars reported in literature [Citation34–38]; (c) adsorption kinetics ; and (d) mass transfer process simulated by weber-morris model].](/cms/asset/706ee523-244b-4662-9550-5512ff7071cd/tcsb_a_2311675_f0002_oc.jpg)
Table 2. Adsorption fitting parameters of C2H6O-BC and p-BC.
Figure 3. Effect of solution initial pH on NOR adsorption onto C2H6O-BC (bichar 2 g L−1, 24 h)(a), co-existing Cu(II) (bichar 1 g L−1, 24 h) (b) and solid-liquid ration (1: 0.5 g L−1; 2: 1 g L−1; 3: 2 g L−1; 4: 4 g L−1, 24 h) (c).
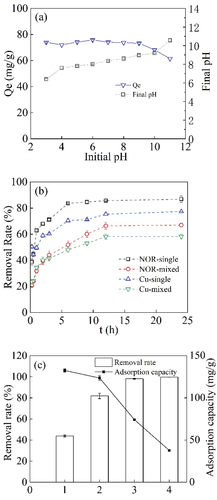
Figure 4. Fixed bed adsorption of NOR adsorption by C2H6O-BC [(a) sorption of NOR with influent concentration of 15 mg L−1 (NOR1), 100 mg L−1 (NOR2) and 200 mg L−1 (NOR3); (b) Thomas model fitting; (c) Yoon-nelson Model fitting; (d) effects of high concentration of Cu(II) (15 mg L−1 NOR +100 mg L−1 Cu); (e) effects of Cu(II) preloading (200 mg L−1 NOR after preloading of 120 mg L−1 Cu); and (f) co-transport of 200 mg L−1 NOR +120 mg L−1 Cu as a comparison for preloading experiment].
![Figure 4. Fixed bed adsorption of NOR adsorption by C2H6O-BC [(a) sorption of NOR with influent concentration of 15 mg L−1 (NOR1), 100 mg L−1 (NOR2) and 200 mg L−1 (NOR3); (b) Thomas model fitting; (c) Yoon-nelson Model fitting; (d) effects of high concentration of Cu(II) (15 mg L−1 NOR +100 mg L−1 Cu); (e) effects of Cu(II) preloading (200 mg L−1 NOR after preloading of 120 mg L−1 Cu); and (f) co-transport of 200 mg L−1 NOR +120 mg L−1 Cu as a comparison for preloading experiment].](/cms/asset/07942070-4478-4c56-9a31-2d5121acedb1/tcsb_a_2311675_f0004_oc.jpg)
Table 3. Removal ratios of nor by C2H6O-BC via STBR.
Supplemental Material
Download MS Word (348.8 KB)Data availability statement
Data will be made available on request.

![Figure 5. The XPS spectrum of C2H6O before and after adsorption [(a) the full XPS spectrum and (b) the high-resolution XPS spectrum of C1s, O1s and Cu2p].](/cms/asset/d7813eab-2cfb-482d-aca6-91655828dab4/tcsb_a_2311675_f0005_oc.jpg)