Figures & data
Figure 1. Structural formulas of mono- and di-methacrylate monomers. Commonly used constituents of dental composites are UDMA (urethane dimethacrylate), Bis-GMA (bisphenol A-glycidyl methacrylate) and TEGDMA (triethylene glycol dimethacrylate). HEMA (2-Hydroxylethyl methacrylate) is found in dental adhesives as part of the resin based dental filling, and EMA (ethyl methacrylate) as an impurity in dental adhesives [Citation75] and positive patch test to EMA has been used to confirm MMA (methyl methacrylate) denture base allergy [Citation5].
![Figure 1. Structural formulas of mono- and di-methacrylate monomers. Commonly used constituents of dental composites are UDMA (urethane dimethacrylate), Bis-GMA (bisphenol A-glycidyl methacrylate) and TEGDMA (triethylene glycol dimethacrylate). HEMA (2-Hydroxylethyl methacrylate) is found in dental adhesives as part of the resin based dental filling, and EMA (ethyl methacrylate) as an impurity in dental adhesives [Citation75] and positive patch test to EMA has been used to confirm MMA (methyl methacrylate) denture base allergy [Citation5].](/cms/asset/e4e2da0d-704c-4067-b821-d4a72e4e5b1d/iabo_a_2223223_f0001_b.jpg)
Figure 2. The antioxidant effect of glutathione is briefly outlined in Figure (A). The antioxidant capacity is mediated by oxidizing the redox-active thiol group (2 GSH -> GSSG) as glutathione reduces target molecules. GSSG (glutathione disulfide) is recycled to GSH in a reaction catalyzed by glutathione-disulfide reductase (GSR). Protection against oxidative stress by this system depends on the maintenance of the GSH concentration. Figure (B) shows the suggested mechanism that results in increased ROS in methacrylate-exposed cells. Methacrylates cause GSH depletion by direct binding to GSH. The reduced antioxidant capacity (red arrows) may initiate a redox imbalance. Oxidative damage on cellular macromolecules may result from this imbalance.
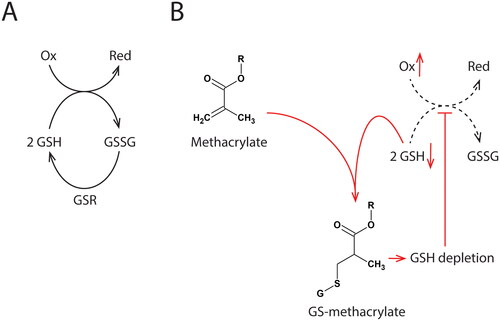
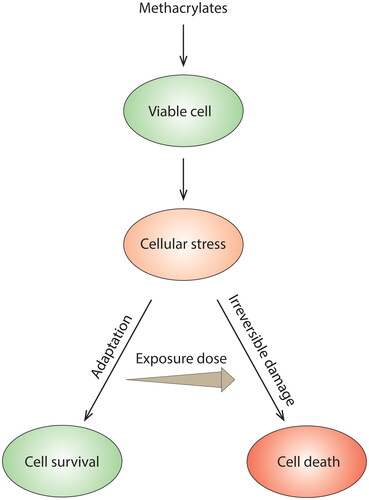
Figure 3. Methacrylate toxicity studies performed in vitro commonly use higher monomer concentrations than those measured in the clinic. At these concentrations, irreversible damage and cell death can occur. Severe cell damage and cell death signaling can potentially overshadow other cellular events of importance for biocompatibility. Focusing on doses below the threshold of cell death may reveal such events.