Figures & data
Table 1. Basic characteristics of KCNH2 missense variants analyzed in this study.
Table 2. Shows pathogenic predictions scores and amino acid changes of all three CA linked KCNH2 mutaions.
Figure 1. Molecular view of built KCNH2 model (a) cytosolic N-terminal PAS domain (b) voltage gated transmembrane domain (VAS) (c) cytosolic C-terminal domain.
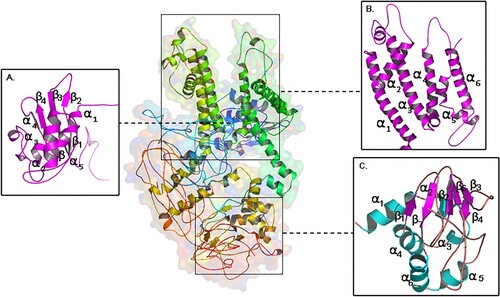
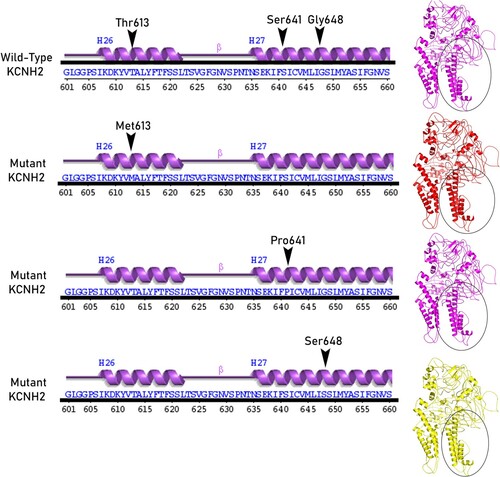
Figure 2. Shows the superpose simulation of both wild type KCNH2 against its mutated protein models, with description of the amino acid changes and their RMSD values at the position of the all mutations.
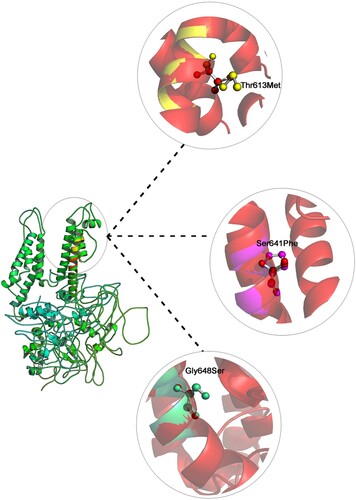
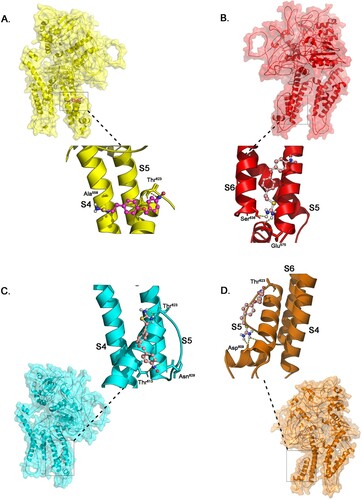
Figure 4. Illustration of the docking simulation combining all KB-R7943 with KCNH2 with (a) wild type protein model (b) mutant Thr613Meth protein model (c) mutant Ser641Phe protein model (d) mutant Gly648Ser protein model.