Figures & data
Table 1. Structure and classification of glycans.
Table 2. Serum/plasma glycans and glycoproteins as cancer biomarkers.
Table 3. Examples of TACA overexpressed due to upregulation of sialyltransferases and fucosyltransferases.
Figure 1. Cancer-associated glycans/tumor-associated carbohydrate antigens (TACAs). TF, the disaccharide Thomsen-Friedenreich antigen; Tn, the monosaccharide Thomsen-nouvelle antigen; sTF, sialylated TF antigen; sTn, sialylated Tn antigen; SSEA-1, stage-specific embryonic antigen-1.
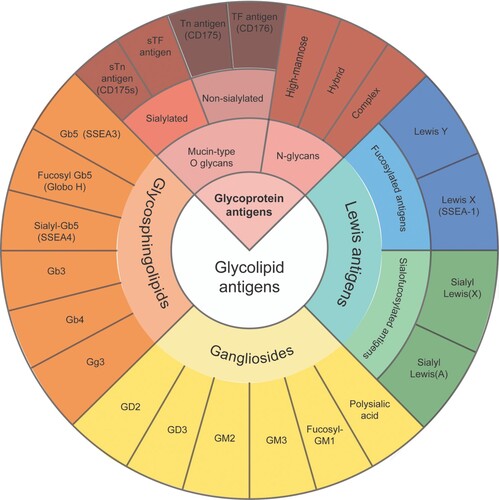
Table 4. Selected cancer associated-glycans expressed in several types of human cancers.
Figure 2. The effect of P-selectin antagonism. The upregulation of P-selectin on tumor associated vasculature (in contrast to E-selectin) may be less about induction of survival and regeneration of malignant cells but more as a factor directly contributing to metastatic cell homing. The simultaneous increased expression of P-selectin on endothelial cells induced by inflammatory cytokines in conjunction with increased expression of P-selectin ligands induced by aberrant glycosylation renders them hyperadhesive. By blocking P-selectin ligands, engineered lectins or glycomimetics can relocate AML leukemic stem cells (LSCs) to the peripheral blood followed by their elimination by chemotherapy. Thereferofer, P-selectin blockade can predictably prevent therapy resistance.
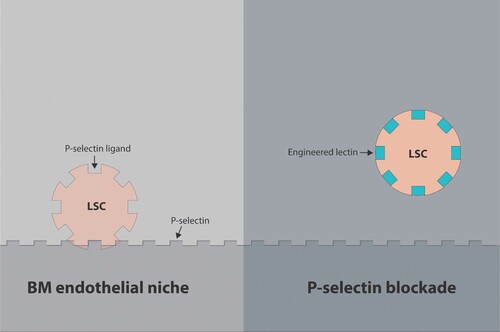
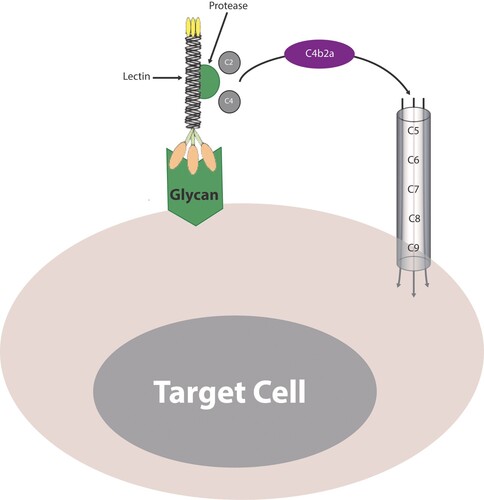
Figure 3. The lectin-protease conjugate. Binding of a lectin-protease conjugate to membrane glycans activates the lectin pathway in a manner similar to the classic pathway where cleaving both C4 and C2 generates the C3 convertase (C4b2a). The C3 convertase cleaves C3 to give rise to a smaller C3a fragment and a larger C3b fragment. The C3b molecules associate with C4bC2a or C3bBb complexes to form the C5 convertase (not shown). The C5 convertase cleaves C5 and initiates activation and assembly of the terminal components, C5, C6, C7, C8, and C9, into the C5b-9 membrane attack complex (MAC) on the surface of target cells.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
