Figures & data
Figure 1. Highly organized structure of skeletal muscle. Individual muscles are surrounded by a layer of connective tissue called the perimysium. Each muscle is composed of bundles of contractile cells termed muscle fibers. These fibers contain sarcomeres, which are repeating units of myofibrils that give skeletal muscle its banding pattern. Two contractile proteins, myosin and actin, form the thick and thin filaments of the sarcomere, respectively. The interaction between myosin and actin produces muscle contraction.
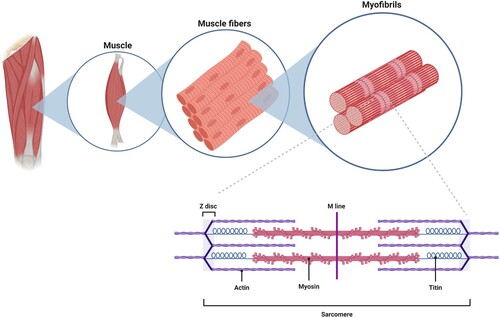
Table 1. Culture strategies to enhance iPSC differentiation into myogenic precursor cells.
Figure 2. Schematic of iPSC-based cell therapy for DMD patients. iPSCs are generated from a tissue biopsy, and the genetic mutation in dystrophin corrected. After expansion in culture to generate sufficient cell numbers for transplantation, the cells are then induced to enter the myogenic pathway to yield cultures of muscle precursor cells. These cells are then transplanted into the patient’s skeletal muscle and produce dystrophin positive myofibers and satellite cells.
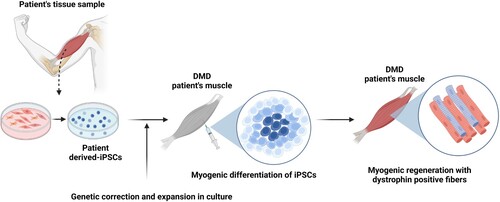
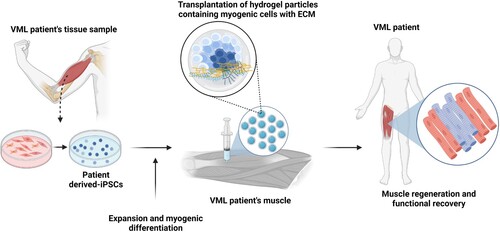
Figure 3. Schematic of iPSC-based cell therapy for VML patients. Similar to DMD patients, a tissue biopsy is used to generate iPSCs, which are then expanded in culture and induced to enter the myogenic pathway to produce muscle precursor cells. Alternatively, culture conditions may be modified to yield cultures containing multiple cells types found in skeletal muscle, such as motor neurons and immature myotubes. Due to the loss of the connective tissue scaffold in VML, a biomaterials-based matrix is transplanted with the iPSC-derived cells to support skeletal muscle regeneration. The transplanted cells utilize the artificial matrix enter myogenesis and effectively replace the loss muscle tissue.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
