Figures & data
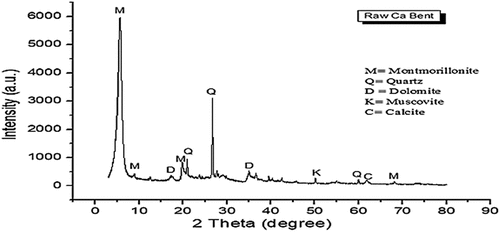
Table 1. XRF results for the raw Montmorillonite sample
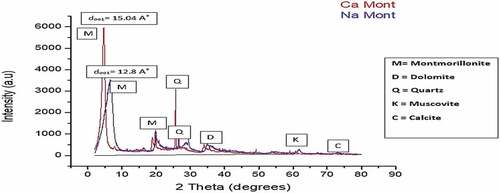
Table 2. XRF results of both Raw and Na-Activated and purified clay
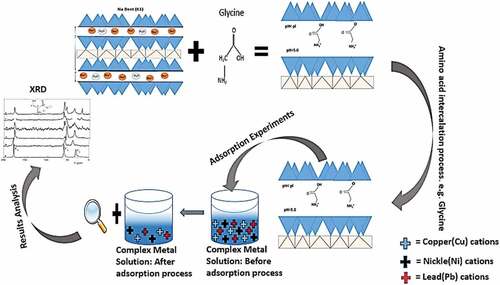
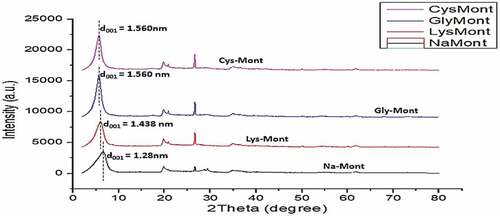
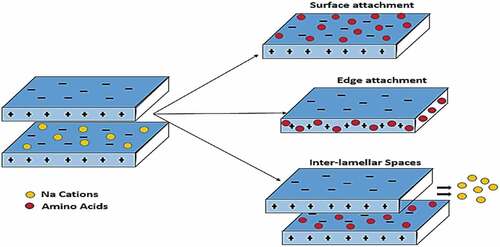
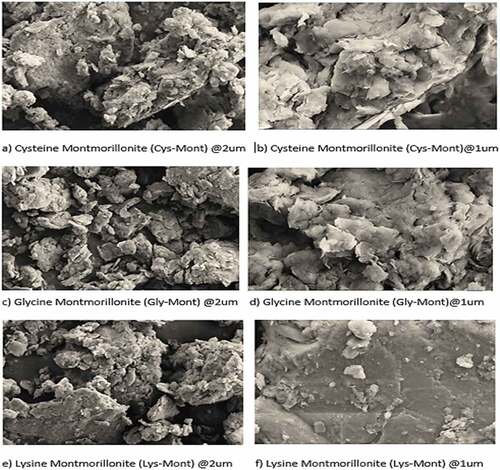
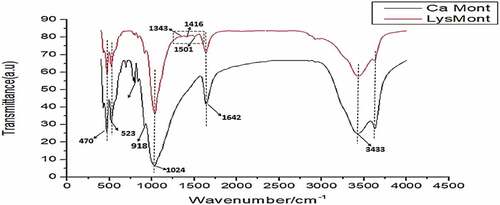
Table 3. Characterization data of both the raw, sodium activated and the amino-acid intercalated clay materials at different pH
Figure 9. Total Heavy Metal removal efficiency for the Amino Acid modified Montmorillonites (AA-Monts) at adsorbent dosage (25 mg—400 mg), pH(6), Shaker speed (180 rmp) and temp. (25°C).
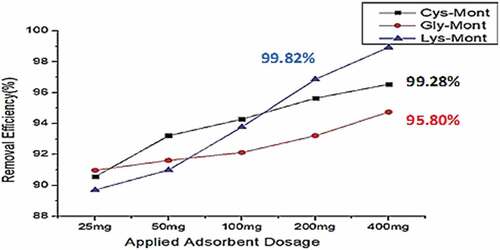
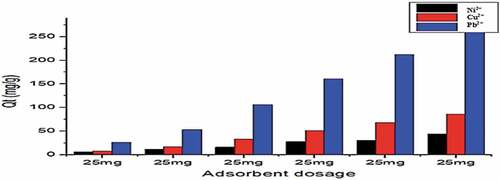
Figure 10. Adsorption of Cu2+, Ni2+, and Pb2+ by Cys-Mont at adsorbent dose range (20 mg-400 mg) pH(6), Shaker speed (180 rmp) and temp. (25°C).
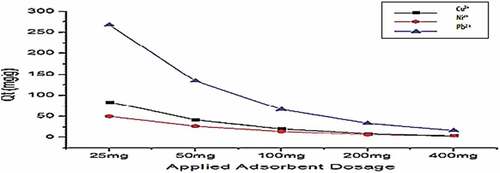
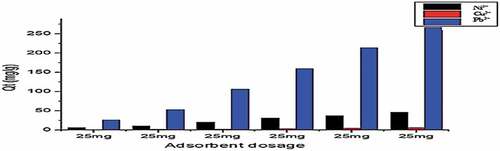
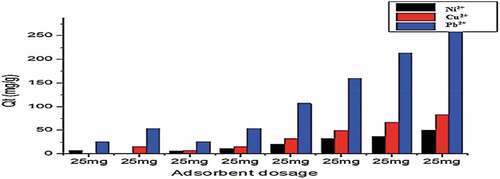
Figure 11. Adsorption of Cu2+, Ni2+, and Pb2+ by Gly-Mont at adsorbent dose range (20 mg-400 mg) pH(6), Shaker speed (180 rmp) and temp. (25°C).
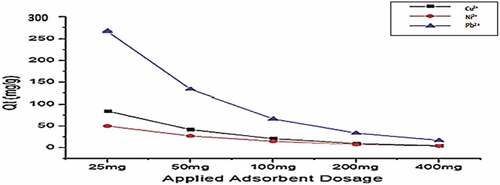
Figure 12. Adsorption of Cu2+, Ni2+, and Pb2+ by Lys-Mont at adsorbent dose range (20 mg-400 mg) pH(6), Shaker speed (180 rmp) and temp. (25°C).
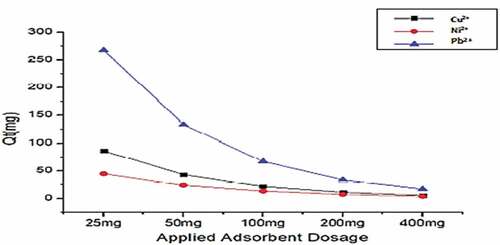
Figure 13. Adsorption capacity (Qt) with increasing initial concentration for the Amino acid-modified Montmorillonite Cys-Mont at adsorbent dose range (25 mg), pH (6), Shaker speed (180 rmp) and temp. (25°C).