Figures & data
Figure 1. Typical EPR spectra of oxidized hemoglobin. The lower curve is magnified 8.3 times (upper curve) to show all the peaks in the region between 500 and 4500 Gauss.
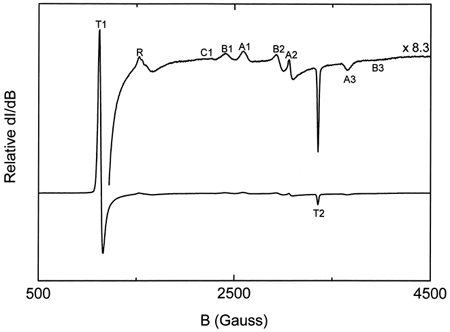
Figure 2. Relative intensities of the EPR high-spin tetragonal metHb complex spectra for ααHb autooxidation at different times.
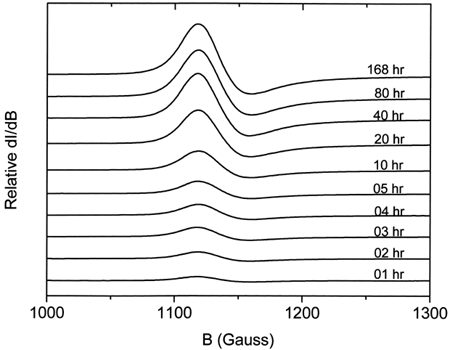
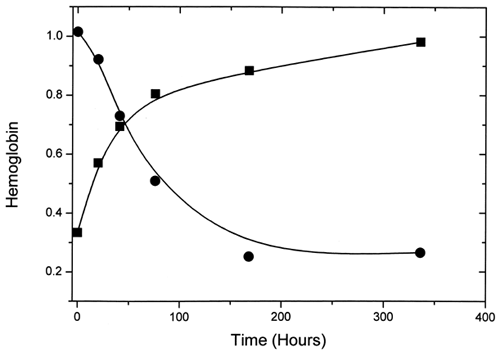
Figure 3. First-order exponential curve fits for ααHb (▪) and LEH (•) for the EPR experiments. Fitted curves have been time-adjusted so that the zero time coincides with metααHb(0).
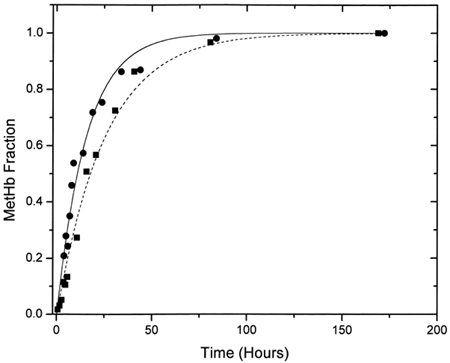
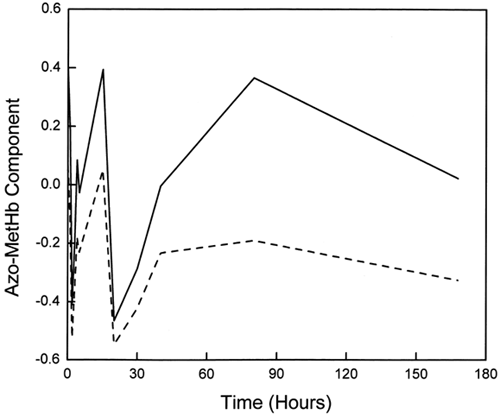
Figure 4. Comparison of the low-spin B1 complex for ααHb (solid line) and LEH (dashed line) showing the shift in the B1 band and the increased intensities for LEH hemoglobin. B1 bands represent those for the 40-h period and have been normalized with respect to the g = 6.0 T1 high-spin bands.
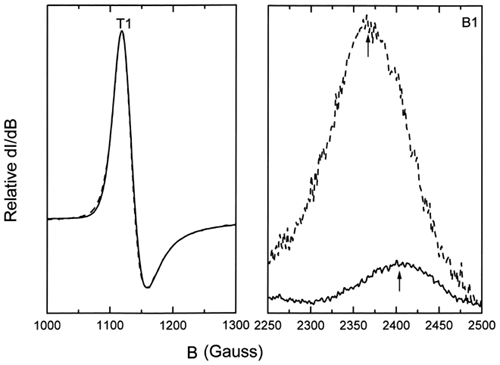
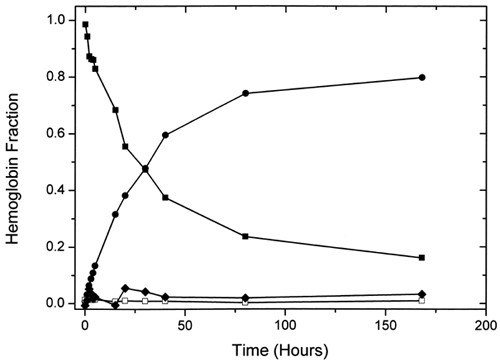
Figure 5. Plot of the variation of the amounts of the different hemoglobin species with time during ααHb autooxidation as determined by multicomponent fits of their UV-vis spectra. Fitted species are oxy (▪), met (•), deoxy (□), and azomet-like (♦) hemoglobin.