Figures & data
Figure 1. Hemolysis (%) of HEMA, TEGDMA and MMA as a function of concentration. TEGDMA (▵), MMA (□), and HEMA (○). HEMA (![]()
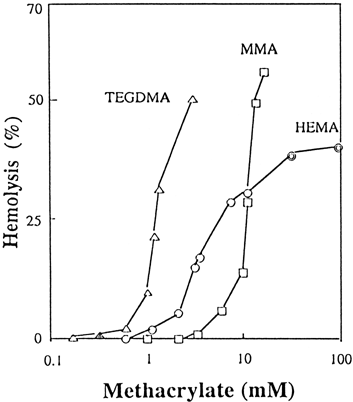
Figure 2. The viability (%) of HEMA, TEGDMA and MMA against HGF cells (a) or HSG cells (b) as a function of concentration. HEMA (▪), MMA (•), and TEGDMA (▴). Values are presented as the mean ±SD of 8 different determinations. The IC50 of TEGDMA against both HSG and HGF was approximately 0.33 mM. That of HEMA against HSG and HGF was approximately 2.10 and 5.60 mM, respectively. The % viability curves of the methacrylate response, the responses of HEMA, TEGDMA and MMA were significantly different (p<0.01) in both HSG (a) and HGF (b) cells as determined by two-way analysis of variance (ANOVA).
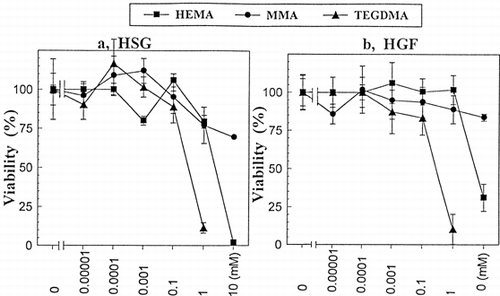
Figure 3. Typical DSC phase-transition profiles for dipalmitoyl phosphatidylcholine/cholesterol (DPPC/CS) liposomes doped with methanol (MEOH), 2-hydroxyethyl methacrylate (HEMA), triethylene glycol dimethacrylate (TEDGMA), or methyl methacrylate (MMA) in phosphate buffer, pH 6.8. (A) Effect of CS on DPPC liposomes: CS was added to DPPC in chloroform and then, DPPC/CS liposomes were prepared as described in the text. (a) DPPC liposomes without CS (control): a main phase-transition peak at 40.5°C and *pretransition peak at 33.5°C, (b) DPPC/CS (10:1, molar ratio): abolishing of the pretransition peak, and (c) DPPC/CS (4:1): broadened DSC; (B) Effect of MEOH on DPPC/CS (4:1) liposomes: (d) 12.5% MEOH, (e) 25% MEOH, and (f) 50% MEOH; (C) Effect of HEMA on DPPC/CS (10:1) liposomes in 12.5% MEOH: (g) 5 mM HEMA, (h) 25 mM HEMA, and (i) 50 mM HEMA; (D) Effect of MMA or TEGDMA on DPPC/CS (10:1) liposomes in 12.5% MEOH; (j) DPPC/CS (10:1) liposomes, (k) 140 mM MMA, (l) 50 mM TEGDMA, and (m) 140 mM TEGDMA.
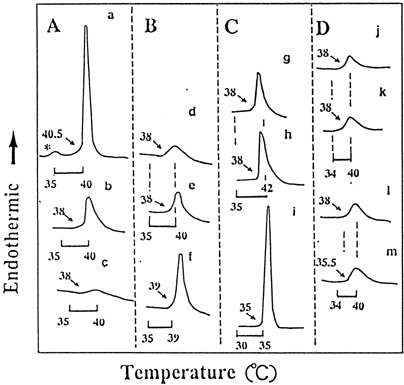
Figure 4. Phase-transition temperature (°C)(A) and height/half height-width (H/HHW) (B) of DPPC liposomes with HEMA (▵) or MMA (○) and TEGDMA/DPPC liposomes (□), as a function of the concentration of monomers in phosphate buffer solution at pH 6.8. An appropriate concentration of HEMA or MMA was added to DPPC liposomes. TEGDMA/DPPC liposomes were prepared in a similar manner to that of DPPC/CS liposomes in Materials and Methods due to the high hydrophobicity of TEGDMA. Values presented are the mean of two different determinations. Computational error for Tm or H/HHW was ≦ ca 5%. The relative H/HHW values = (H/HHW of specimen/ H/HHW of control) × 100.
Table I. Phase Transition Temperature (Tm) and Enthalpy (ΔH) of DPPC, DPPC/CS Liposomes with or without Methanol (MEOH), HEMA, or TEGDMA in Phosphate Buffer Solution at pH 6.8#
Figure 5. 1H-NMR spectra of HEMA/DPPC (1:1 molar ratio), TEGDMA/DPPC (1:1, molar ratio), and DPPC liposomes in deuterium oxide (D2O), as a function of temperature at 30°C and 52°C (A) and the chemical structure of triethylene glycol dimethacrylate (TEGDMA), 2-hydroxyethyl methacrylate (HEMA), and L-α-dipalmitoyl phosphatidylcholine (DPPC), and their numbering system (B). HEMA/DPPC: The indicated concentration of HEMA was added to DPPC liposomes; TEGDMA/DPPC: TEGDMA/DPPC liposomes were prepared in a similar manner to that of DPPC/CS liposomes in Materials and Methods; DPPC: DPPC liposomes were prepared as described in Materials and Methods. After preparation, the specimens were allowed to equilibrate for 6 h at 5°C before they were measured. X, y, and z is are the terminal methyl, acyl chains and choline methyl group of DPPC, respectively(B). TMSPA was used as an external reference in deuterium oxide and the chemical shifts are ppm downfield from TMSPA. DHO, deuterium oxide contained very small quantities of H2O.
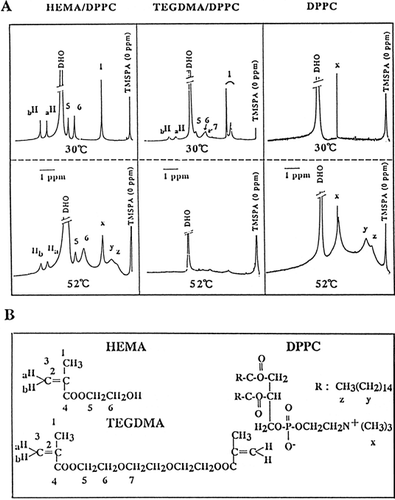
Table II. The Chemical Shift Differences (Footnote#