Figures & data
Figure 1. Hill plots for intraerythrocytic α-nitrosyl Hb (closed symbols) and untreated erythrocytes (open symbols) at pH 5.8 (A) and pH 7.4 (B). Experimental conditions were as follows: Treated and α-nitrosyl Hb-containing erythrocytes were suspended in 0.15 M sodium-potassium phosphate buffer of appropriate pH, in an amount equivalent to 60 μM heme (untreated erythrocytes) or 120 μM heme (α-nitrosyl Hb-containing erythrocytes). Temperature was 15°C. Deoxygenation was monitored using a dual wavelength spectrophotometer by tracing the optical absorbance increase at 577 nm referenced against 700 nm.
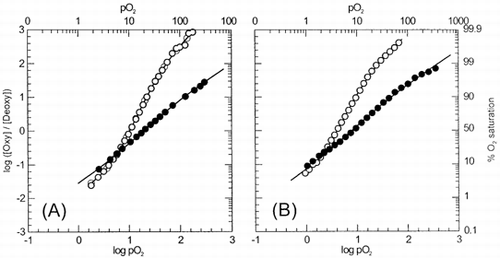
Figure 2. pH dependence of the oxygen affinity (as expressed by P50 values) of α-nitrosyl Hb and untreated Hb within erythrocytes (closed symbols) and in solution (open symbol). (•), intraerythrocytic α-nitrosyl Hb; (▪), untreated erythrocytes; (○), α-nitrosyl Hb (□), α- nitrosyl Hb + DPG, and native HbA (broken line). Compared to in solution conditions, Bohr effect is reduced in intraerythrocytic α-nitrosyl Hb (from –0.86 to –0.31 protons per binding site). Experimental conditions as in . Data for solution conditions were taken from Citation[[1]]
![Figure 2. pH dependence of the oxygen affinity (as expressed by P50 values) of α-nitrosyl Hb and untreated Hb within erythrocytes (closed symbols) and in solution (open symbol). (•), intraerythrocytic α-nitrosyl Hb; (▪), untreated erythrocytes; (○), α-nitrosyl Hb (□), α- nitrosyl Hb + DPG, and native HbA (broken line). Compared to in solution conditions, Bohr effect is reduced in intraerythrocytic α-nitrosyl Hb (from –0.86 to –0.31 protons per binding site). Experimental conditions as in FIGURE 1. Data for solution conditions were taken from Citation[[1]]](/cms/asset/3efbf5a6-6f58-4401-a5b6-38dc1d1bd2f8/ianb19_a_11116782_uf0002_b.gif)
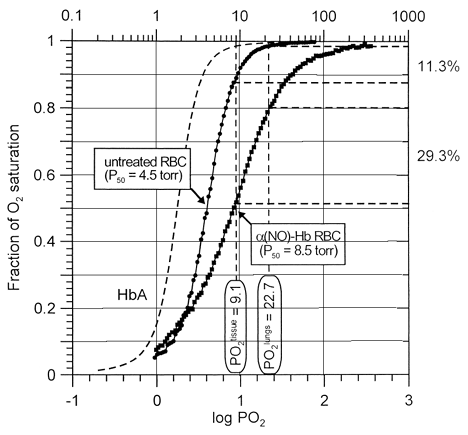
Figure 3. Oxygen delivery performance of untreated DPG-depleted (circles) and α-nitrosyl Hb-containing erythrocytes (squares) at pH 7.4 and 15°C, as revealed by comparing their oxygen dissociation curves. Normal Hb carries four molecules of oxygen per tetramer, whereas α-nitrosyll Hb half of this amount, since both the α-subunits are ligated with NO. While under normal conditions expired erythrocytes (2,3-DPG-depleted cells) unload about 11% of their total oxygen loading capacity going from lungs to tissues, α-nitrosyl Hb-containing erythrocytes deliver 29%. Data correspond to respective curves shown in .