Figures & data
Figure 57. Schematic representation of principle of conventional artificial kidney (hemodialyzer) (left), and artificial cells for artificial kidney (right). (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
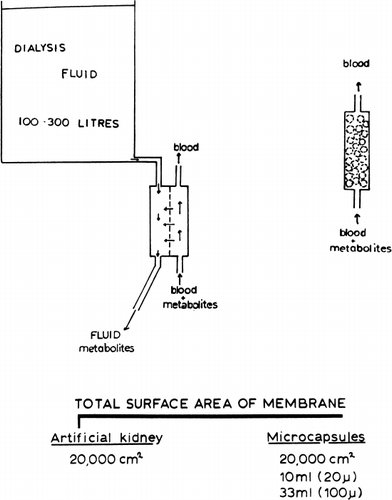
Figure 58. Extracorporeal shunt containing urease-loaded artificial cells. Effect on systemic arterial blood ammonia and blood urea level. (From Chang, 1966. Courtesy of the American Society for Artificial Internal Organs.)
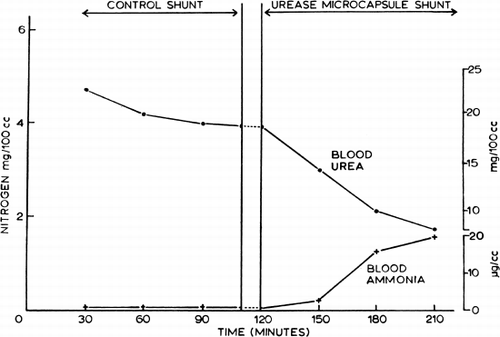
Figure 59. The length of column required for f=1/10 is plotted versus artificial cell diameter. P=1×10−4 cm/sec in curve 1, and P=5×10−5 cm/sec in curve 2. Other details are described in the test. (From Levine and LaCourse, 1967. Courtesy of Interscience Publishers, New York.)
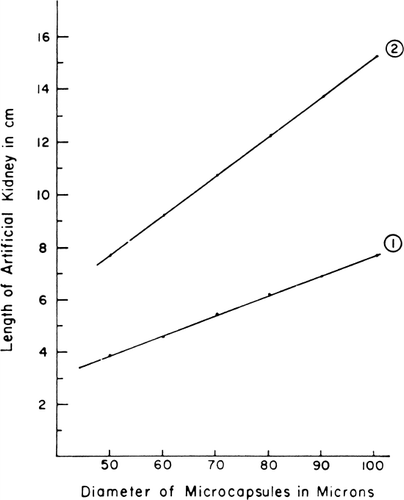
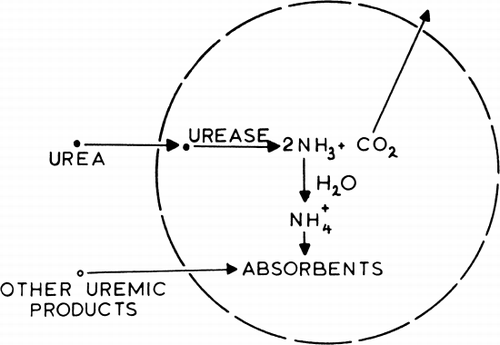
Figure 60. Schematic representation of the action of urease-loaded artificial cells in the gastrointestinal tract.
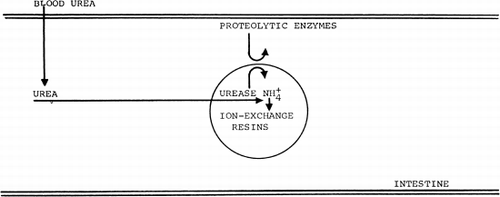
Figure 61. Extracorporeal shunt chamber containing 300 gm of ACAC articial cells used for in vivo experiments and for clinical trials. (From Chang et al., 1970. Courtesy of the American Society for Artificial Internal Organs.)
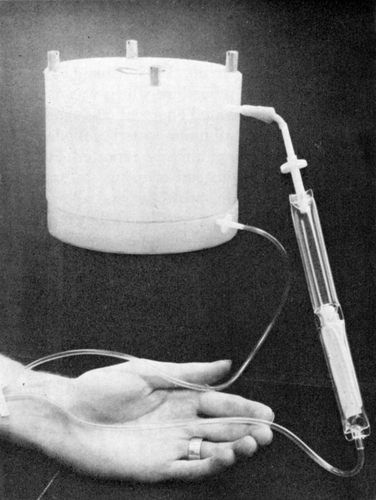
Figure 62. Rate of decrease in concentration of different solutes in stirred batch experiments (equivalent to 300 gm ACAC artificial cells in 9 liters of solution). (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
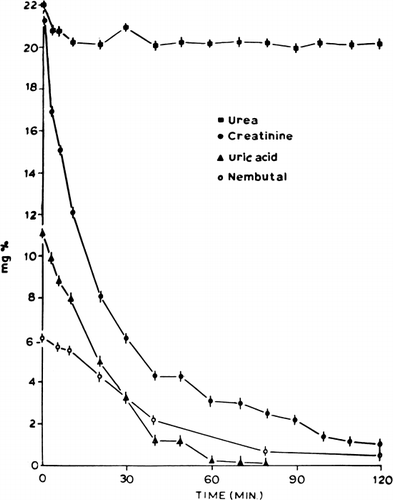
Figure 63. In vitro creatinine clearance of a shunt containing 300 gm of ACAC artificial cells. (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
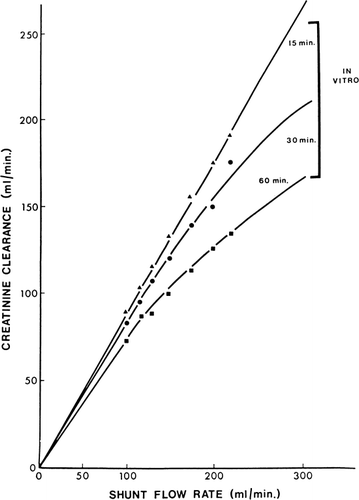
Figure 64. Schematic representation of the removal of creatinine by 300 gm of ACAC artificial cells.
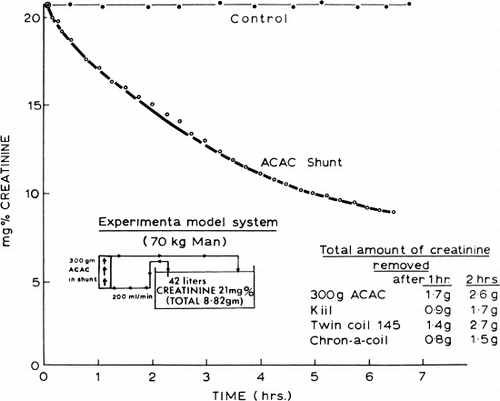
Figure 65. Rate of change of plasma creatinine levels in nephrectomized dogs treated with hemoperfusion (blood flow 120 ml/min) through free AC activated charcoal, CAC artificial cells, and ACAC artificial cells; 300 gm in each shunt. Lower curve (○) represents rate of change of plasma creatinine with higher blood flow (300 ml/min) through 300 gm of ACAC artificial cells. (From Chang and Malave, 1970. Courtesy of American Society for Artificial Internal Organs.)
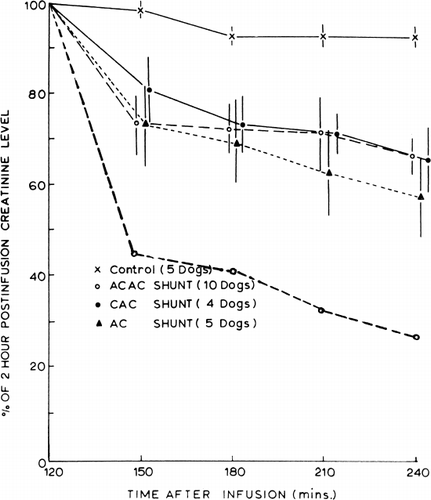
Figure 66. Effects of two-hour hemoperfusion through 300 gm of ACAC artificial cells on plasma creatinine levels of 10 nephrectomized dogs. (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
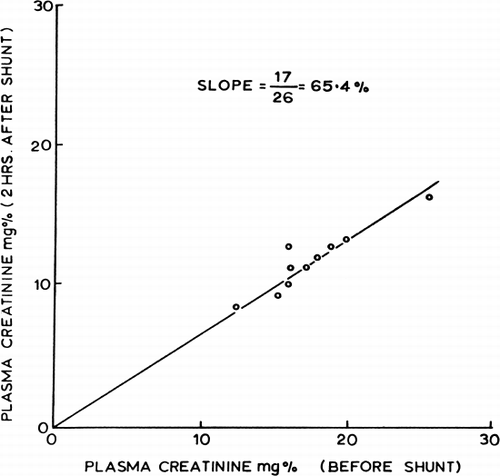
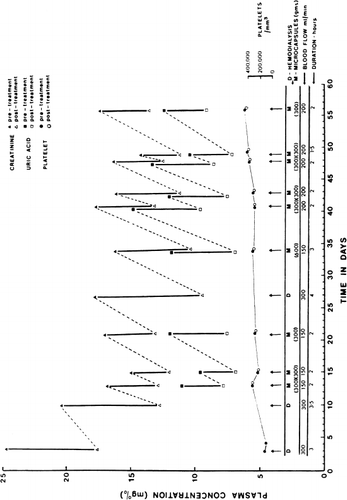
Figure 67. Response of patient (B.B.) treated with hemoperfusion through 300 gm of ACAC artificial cells. (From Chang and Malave, 1970. Courtesy of the American Society for Artificial Internal Organs.)
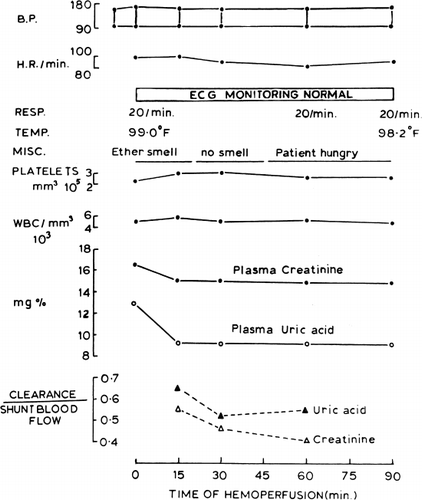
Figure 73. Internal resistance and creatinine clearance of microcapsule (ACAC) artificial kidney compared to the values obtained by Cestero et al. for other hemodialyzer. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
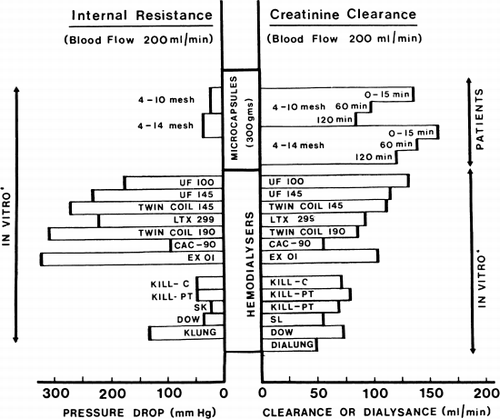
Figure 68. Creatinine clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in 15 clinical trials. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
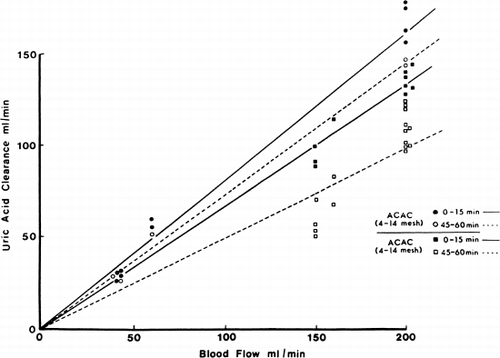
Figure 69. Uric acid clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in 15 clinical trials. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
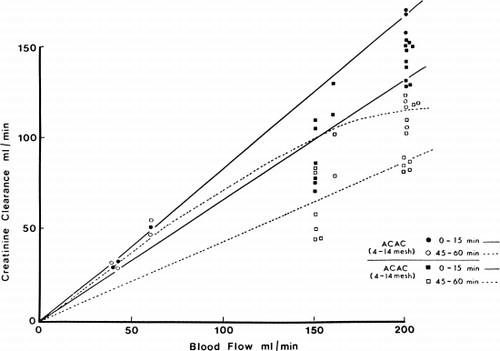
Figure 70. Creatinine clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in clinical trials compared to those obtained using EX01 hemodialyzers. Blood flow rate 200 ml/min. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
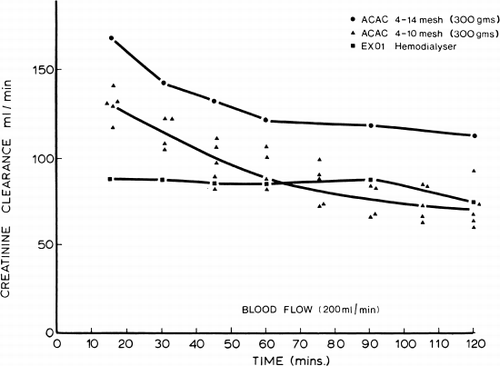
Figure 71. Uric acid clearance of 300 gm of albumin-collodion coated activated charcoal (ACAC) in clinical trials compared to those obtained using EX01 hemodialyzers. Blood flow rate 200 ml/min. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)
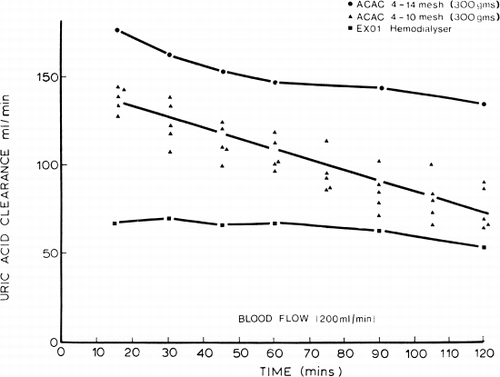
Table 5. Removal of Guanidine
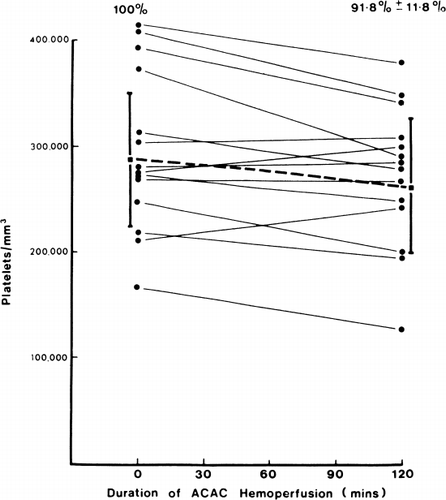
Figure 72. Effects of hemoperfusion over albumin-collodion coated activated charcoal on patients' arterial platelet levels. (From Chang et al., 1971. Courtesy of the American Society for Artificial Internal Organs.)