Figures & data
Figure 1. Molecular weight distributions of two types of polyhemoglobin. PolyHb 10:1 prepared using a molecular glutaraldehyde to hemoglobin ratio of 10 to 1; PolyHb 17:1 using a ratio of 17 to 1.
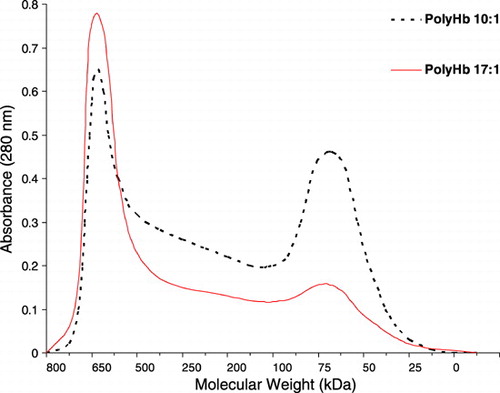
Figure 2. The maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level when using the 2 types of polyHb from .
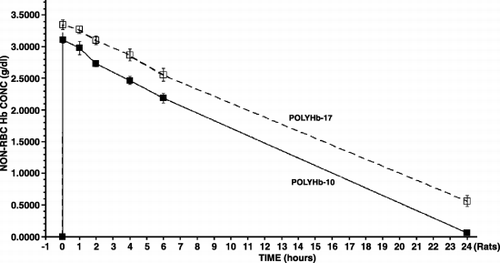
Figure 3. The type of polyHb used for nanoencapsulation and the maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level.
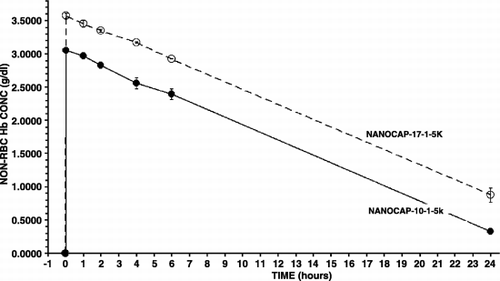
Figure 4. Variations in the molecular weights of polylactic acid (5 K or 15 K) used and the amount of polyethylene‐glyco‐polylactide copolymer used (1 or 1.5). Effects on the maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level.
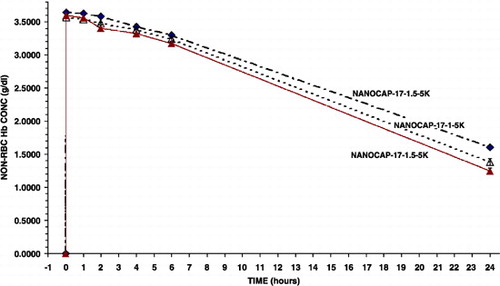
Figure 5. Effects of crosslinking (XL) the newly form nanocapsules on the maximal systemic non‐rbc hemoglobin reached after infusion and the time to reached a given non‐rbc hemoglobin level.
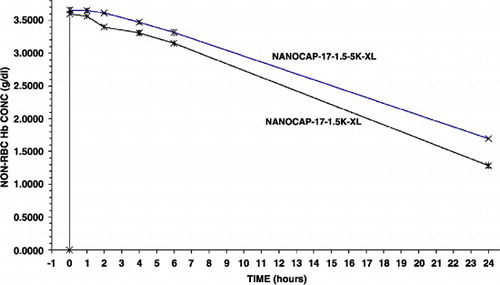
Figure 6. Analysis of results from above. PolyHb‐17 is used as the standard for extrapolation of the results obtained for the different types of Hb nanocapsules. The time for PolyHb‐17 to reach a non‐rbc hemoglobin level of 1.67 gm/dl is 14 hours in rats equivalent to 24 hours in human. The time to reach this non‐RBC hemoglobin concentration of 1.67 gm/dl is used to analyze the time for Hb naoocapsules to reach this level. This is then used to calculate the equivalent time for human.
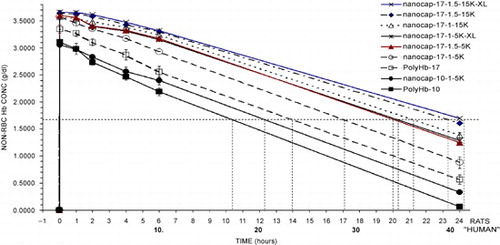
Table 1. Summary from the Analysis of Results
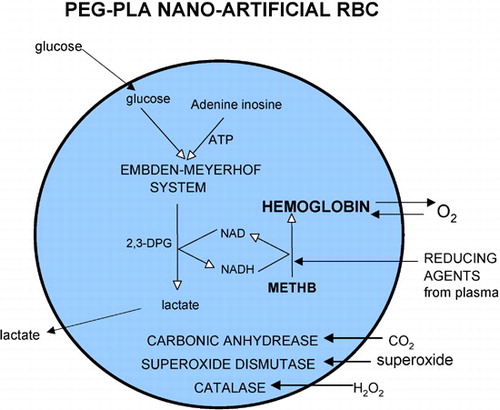
Figure 7. Nanodimension artificial red blood cells containing red blood cell enzymes including methemoglobin reductase system, carbonic anhydrase, catalase and superoxide dismutase. Methemoglobin formation can be reduced by either the more complicated methemoglobin reductase system or by plasma reducing agents diffusing into the nanocapsules.