Figures & data
Figure 1. SDS-PAGE Electrophoretogram of hemoglobin in improved pretreatment process. 1. Bovine hemoglobin standard (Sigma), 2. MW marker, 3. lysate, 4. filtrate of 0.45 µm membranes, 5. retentate of 30 KD membranes.
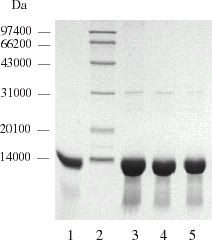
Table 1. Comparison of two pretreatment methods
Figure 2. SDS-PAGE electrophoretogram of hemoglobin chromatography with Q Mustang at various pH values. 1. pH 7.8; 2. pH 7.6; 3. pH 7.4; 4. MW marker; 5. Bovine hemoglobin standard (Sigma).
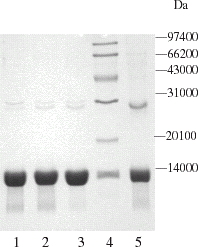
Figure 3. SDS-PAGE electrophoretogram of hemoglobin chromatography with QMA Spherosil LS and Q Sepharose Big Beads at various pH values. 1. Q Big Beads pH 6.4; 2. Q Big Beads pH 6.8; 3. Q Big Beads pH 7.4; 4. Q Big Beads pH 7.8; 5. lysate; 6. ultrafiltrated with 30 KD membrane; 7. QMA pH 7.4; 8. QMA pH 6.8; 9. Marker.
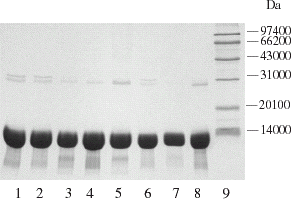
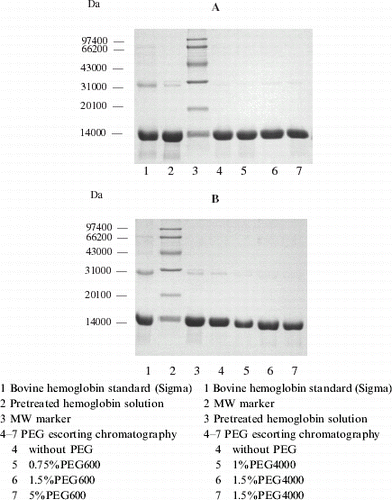
Figure 5. Profile of hemoglobin with PEG600 escorting chromatography. Chromatographic column was packed with 10 mL Q Sepharose Big Beads; equilibrium buffer: 10 mmol L−1 PBS, pH 7.8; loading quantity: 100 μL protein with a concentration of 200 mg mL−1; loading rate: 25 cm h−1, 5 min later the flow rate was raised to 75 cm h−1, rinsed until hemoglobin flow through completely (45 min later), absorbed hemoglobin and impurities were eluted by 10 mmol L−1 PBS containing 1 mol L−1 NaCl at a flow rate of 150 cm h−1.
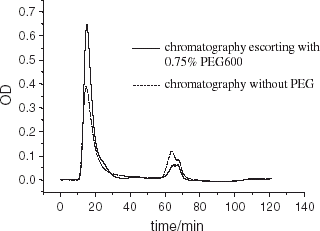
Figure 7. Scanning profile of SDS-PAGE electrophoretogram for purified hemoglobin. (View this art in color at www.dekker.com.)
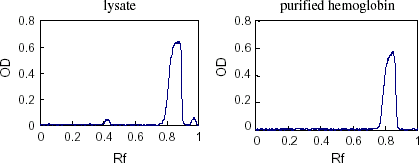
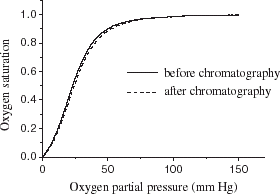
Figure 8. High performance gel filtration liquid chromatography analysis of purified hemoglobin. Chromatography column: TSK 3000SW, 7 μm, 250 × 4.5 mm; mobile phase: 50 mmol L−1 PBS containing 100 mmol L−1 NaCl, pH 7.2: loading quantity: 20 μL, flow rate: 1.0 mL min−1, detected at 280 nm.
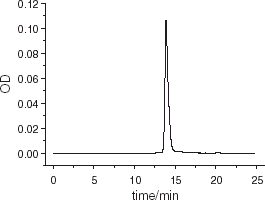
Table 2. Protection effect of various sugar on hemoglobin activity in lyophilizing
Table 3. Bioactivity, purity and recovery of hemoglobin in the optimal process