Figures & data
Figure 1 Schematic drawing of the LC templating synthesis of mesoporous materials. (Reprinted with permission from [Citation44], Nature Publishing Ltd ©1992.)
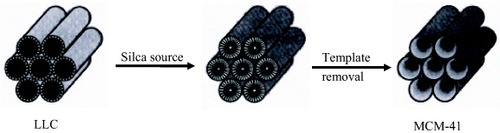
Figure 2 Schematic representation of the templating process used to deposit the H1−e metal films. The cylinders represent the micellar rods in the LLC phase. (Reprinted with permission from [Citation64], American Chemical Society © 2003.)
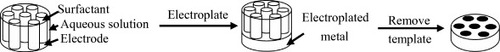
Table 1 The investigated metal/surfactant combinations and as-obtained structures. (Reprinted with permission from [Citation65], Wiley-VCH Verlag GmbH & Co. KGaA © 2006.)
Figure 3 Schematic formation process of the mesoporous hollow microspheres. (Reprinted with permission from [Citation76], American Chemical Society © 2005.)
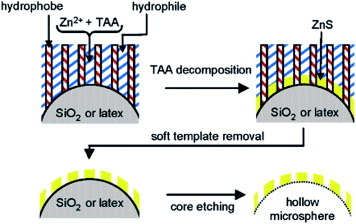
Figure 4 Schematic of the proposed enhanced alignment of reverse hexagonal liquid-crystalline phase during nanowire electrodeposition. (Reprinted with permission from: [Citation96], Wiley-VCH Verlag GmbH & Co. KGaA © 2002.)
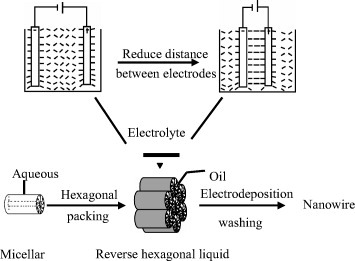
Figure 5 Polarized light micrographs of surfactant AOT systems (×400). AOT/p-xylene/H2O reverse hexagonal liquid crystal before (a) and after (b) doping of Cu2+ ions. (Reprinted with permission from [Citation98], American Chemical Society © 2002.)

Figure 6 SEM images of cuprite nanowires electrodeposited from reverse hexagonal LC phase AOT (1.5 M)/p-xylene/CuCl2 (0.1 M) at deposition potential of −1.1 V with electrode distance of 0.5 mm for different deposition times: (a) 1 h, (b) 2 h and (c) 2 h, at different magnifications. (Reprinted with permission from [Citation98], American Chemical Society © 2002.)

Figure 7 Schematic models for the formation of Pt nanotubes in the mixed surfactant templating system: (a) Mixed (C12EO9/Tween 60) and (b) single (C12EO9) surfactant cylindrical rod like micelles. (c) Pathway from micellar solution to metal nanotubes by the reduction of metals salts confined to the aqueous shell of mixed-surfactant cylindrical micelles. The metal salts and water molecules are omitted from the models. (Reprinted with permission from [Citation100], Wiley-VCH Verlag GmbH & Co. KGaA © 2004.)
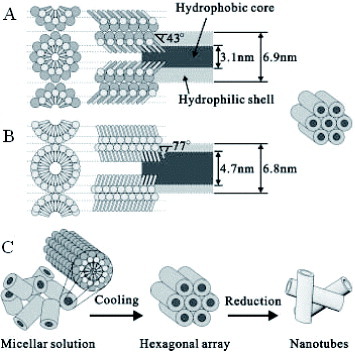
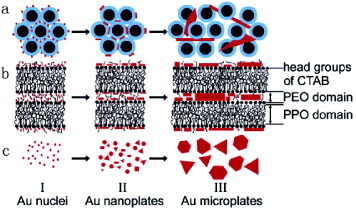
Figure 8 Schematic growth mechanism of Au microplates from P123(45%)–CTAB(1.0%)–HAuCl4 (0.03 M) system: (a) cross section of the hexagonal phase, (b) side view of two adjacent cylinders and (c) growth process. (Reprinted by permission from [Citation105], American Chemical Society © 2005.)