Figures & data
Figure 1 (a) The HOMO–LUMO interchange caused by the strong dimerization. (b) A schematic display of the typical packing mode of the dimers in the β′-type crystals, showing the solid-crossing structure. The definition of the transfer integrals is also indicated.
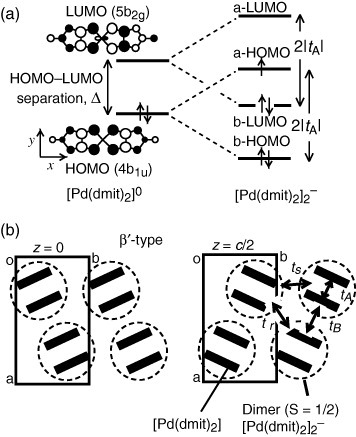
Figure 2 Typical temperature dependence of spin susceptibilities of the triangular Z[Pd(dmit)2]2 (Z=Me4As and Et2Me2P) showing strongly frustrated features, together with the magnetic model (the anisotropic triangular lattice) defined for the real structure. Solid curves indicate the χ values calculated on the basis of the spin-1/2 Heisenberg isotropic (J′=J) triangular lattice model.
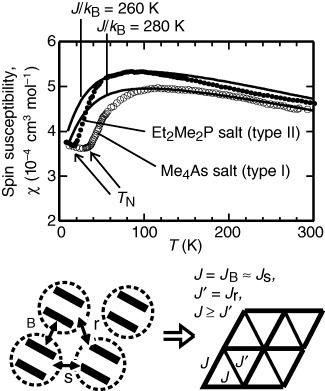
Figure 3 Variety of low-temperature spin states arising from the frustrated spin system on the anisotropic triangular lattice of Z[Pd(dmit)2]2. Depending on the spatial anisotropy and counter cation (Z), unconventional valence bond (VB) states appear at low temperatures. AF LRO is antiferromagnetic long-range order.
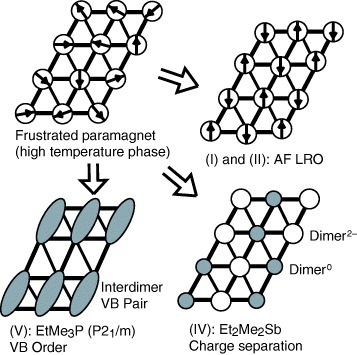
Table 1 Classification of magnetic properties of the triangular Z[Pd(dmit)2]2.
Figure 4 (a) The key parameters controlling the insulator-metal boundary. (b) Schematic phase diagram based on the structure and resistivity measurements under pressure or uniaxial strain. Solid arrows indicate simulated uniaxial effects with a 0.1 Å reduction in the interdimer distance along the corresponding direction. For the Et2Me2P salt, dotted arrow indicates the reduction of about 0.2 Å along the b-axis under the hydrostatic pressure of 0.7 GPa. (Reprinted with permission from [Citation45]. Copyright 2007 Royal Society of Chemistry.) (c) Schematic phase diagram taking account of the temperature dependence of the magnetic behaviour. The Mott transition line corresponds to the metal-insulator boundary in (b). The broken lines denote metal-insulator crossover. The critical point (the end-point of the first-order metal–insulator transition line) is expected to exist slightly above TN, as qualitatively indicated here. It is not well resolved yet, due to experimental difficulty in fine control of pressure in this range. The magnetic susceptibility varies little through the metal-insulator transition because of the strong correlation in the metallic phase [Citation46], which made it difficult to detect the transition by magnetic study.
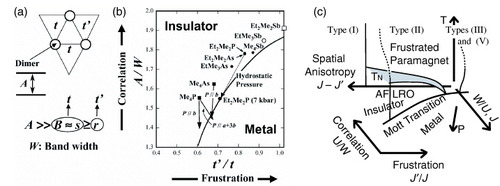
Figure 5 (a) The spin susceptibility of the Et2Me2Sb salt showing the transition at 70 K to the charge-separated phase. (b) The spectral changes in the near-IR region, with the assignment of the split peaks.
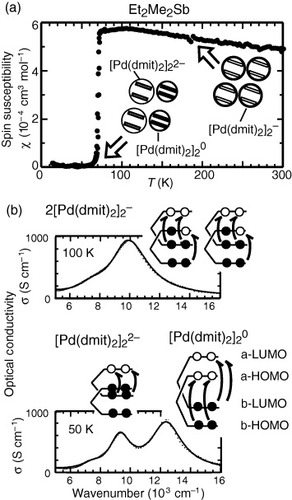
Figure 6 (a) The valence bond ordering as detected by the χ-slope anomaly at 25 K. The discontinuity seen at 30 K has no physical meaning; it is an experimental artefact due to zero-crossing of the raw magnetization signal. (b) Doubled periodicity accompanying the valence bond ordering.
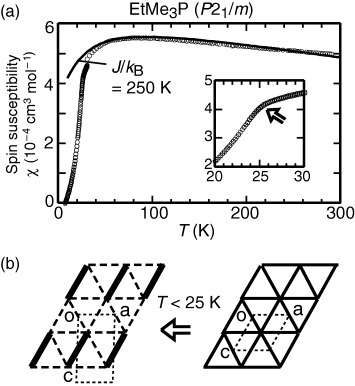
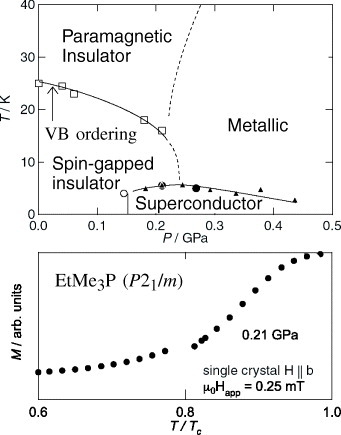
Figure 7 Upper panel: the P–T magnetic phase diagram, in which the pressure-induced superconductivity appears adjoining the insulating phase with the valence bond order. Lower panel: Superconducting diamagnetic signals of the single crystal of the P21/m EtMe3P[Pd(dmit)2]2 [Citation73].