Figures & data
Figure 1 Characterization of the pluripotency of ES cells on artificial extracellular matrix using recombinant fusion protein of E-cadherin and Fc-region of IgG. (a) Leukocyte inhibitory factor (LIF)-dependent activation of STAT3 blocks ES cell differentiation and promotes self-renewal. Oct3/4 is the most important transcription factor in this pathway. (b) Immunocytochemical labeling shows undifferentiated mouse ES cells with positive reactivity for Oct3/4 on artificial ECM (left side) and colony of undifferentiated mouse ES cells on conventional culture system (right side). (c) H&E staining of teratomas as a standard test of pluripotency suggesting the potential of ES cells on artificial matrix into ectoderm, endoderm and mesoderm [Citation7].
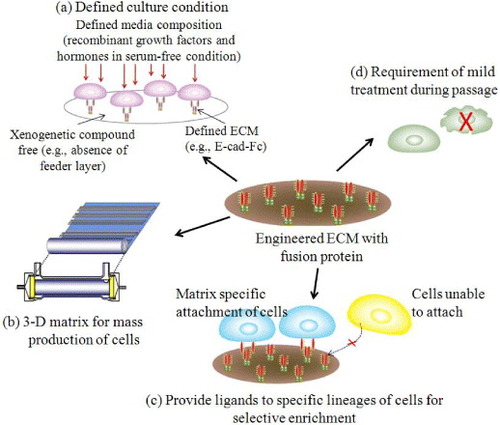
Figure 2 Interactions of stem cells with extracellular microenvironments and its effect on cell behavior. Stem cell fate is influenced by coordinated interaction of soluble factors, extracellular matrix and signals from neighboring cells. Specific binding of signal molecules with cell-surface receptors induces complex intracellular signaling pathways with subsequent effect on gene expression, self-renewal, morphogenesis and differentiation [Citation1, Citation4].
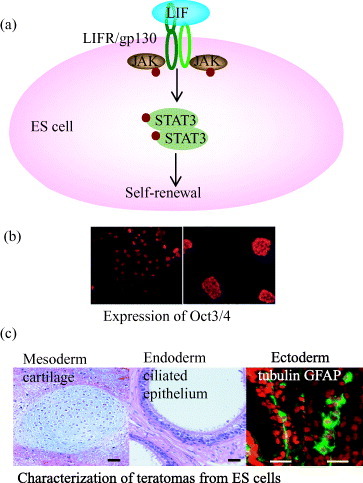
Figure 3 Design strategies for cell–matrix engineering based on our originally proposed concept to induce specific cellular behavior. The receptor-binding domain of naturally occurring proteins can be modified by recombinant technology. The most important components could be integrin dependent ligands (such as collagen, laminin or fibronectin), cell–cell adhesion molecules (such as cadherins or ICAM), binding sites for growth factor proteins (such as EGF, HGF and LIF) or small molecules (such as drugs or hormones) to make it easily accessible to cells, and also ligands for endocytosis (such as LDL, transferrin, or ASGP-R). The use of such recombinant approaches in ECM design may allow matrices to be selective for a specific cell or tissue in a homogeneous environment [Citation3, Citation5–7, Citation19, Citation23, Citation24, Citation26]. LN, laminin; FN, fibronectin; ICAM, intercellular adhesion molecule; LDL, low density lipoprotein.
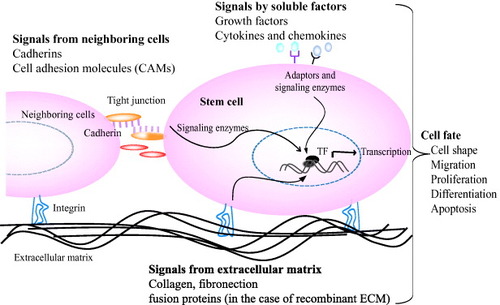
Figure 4 Construction of chimera proteins. Generated segments of an extracellular domain of mouse E-cadherin, leukocyte inhibitory factor (LIF) or epidermal growth factor (EGF) and an IgG-Fc region were subcloned into a eukaryotic expression vector pRC/CMV via HindIII–NotI site and NotI–XbaI site, respectively [Citation5–7, Citation19]. ECM using chimera proteins has specific applications, several of which are listed in (a)–(c).
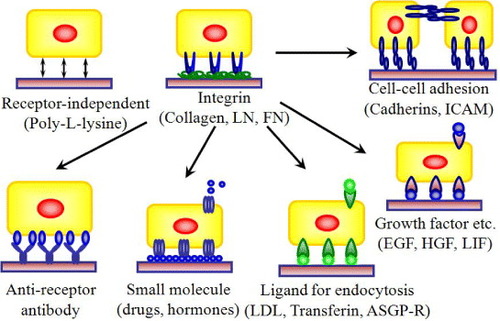
Figure 5 Comparison of two different culture conditions: colony formation and single cell culture. Embryoid body and cells on feeder layer favor the formation of colony of aggregated cells (left side) [Citation30, Citation31]. Cells on matrices (right side) favor single cell culture with homogeneous culture conditions [Citation7]. Specific merits and limitations are enumerated in the figure for these culture conditions. Scale bar: 100 μm.
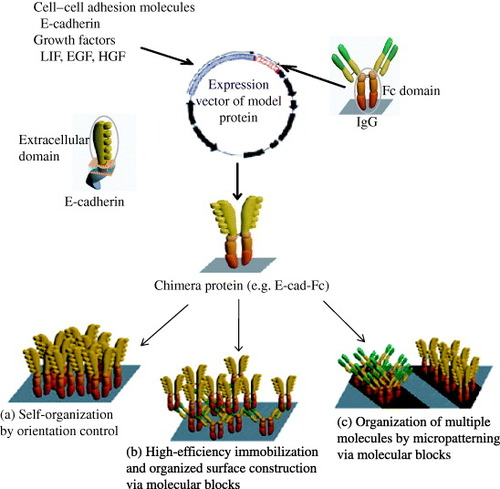
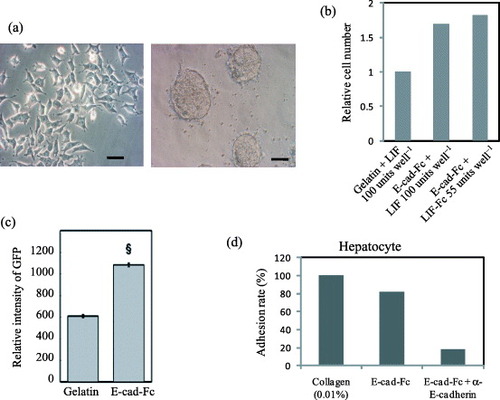
Figure 6 Cell morphology, adhesion and transfection efficiency of mES cells and hepatocytes on the fusion protein-immobilized surfaces. (a) Morphological observation of mES cells (EB3) on a gelatin- or E-cad-Fc-coated surface. Scale bar: 50 μm. (b) The higher proliferative activity of EB3 cells on 5 μg ml−1 of E-cad-Fc coated matrix compared to 0.1% gelatin coated surfaces in the presence of LIF for 2 days. Even at lower concentration of immobilized LIF (LIF-Fc) at 55 units well−1, the ES cells showed higher proliferation ability on a co-immobilized surface than on a gelatinized surface. (c) Transfection efficiency of EB3 cells on two different matrices. The data indicate means±SEM. §:P<0.001 versus gelatinized plates. (d) Primary hepatocytes could adhere to E-cad-Fc-coated dish as well as collagen-coated surface after 4 h [Citation6, Citation7, Citation19].
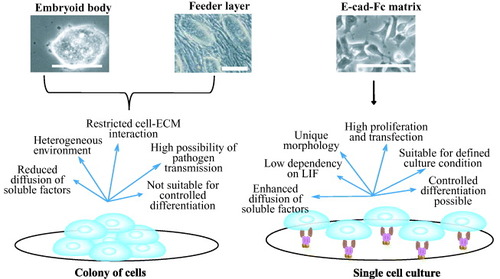
Figure 7 Future prospects of engineered ECM for ES cells. (a) The use of recombinant extracellular matrix together with defined media supplemented with recombinant proteins and elimination of xenogenetic components would be one step forward to develop defined culture condition. (b) Three-dimensional (3D) culture systems using recombinant ECM molecule and different combinations of growth factors and hormones would allow highly efficient and mass production of ES cells for therapeutic applications. (c, d) The guided differentiation of ES cells under fully defined culture condition with lineage-specific matrices would provide the opportunity for genesis of economically feasible, purified and non-stressed cells for proliferation and differentiation.