Figures & data
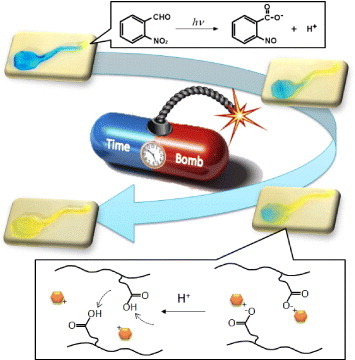
Figure 1 Schematic of a timed explosive drug release from pH-responsive hydrogels by utilizing a phototriggered spatial pH-jump reaction.
Scheme 1 Preparation scheme of P(NIPAAm-co-CIPAAm) gels by a redox polymerization (a). For drug loading, P(NIPAAm-co-CIPAAm) gels were first swollen at room temperature in a solution containing 1 mg ml−1 of DOPA. After 2 days, the gels were rinsed in water to remove non-interacted DOPA. The gels were then immersed in a solution containing 10 mM of o-NBA for 2days (b). For the drug release experiment, one part of the gel (5 × 5 mm2) was immersed in water and another part (5 × 5 mm2) was exposed to UV irradiation (c).
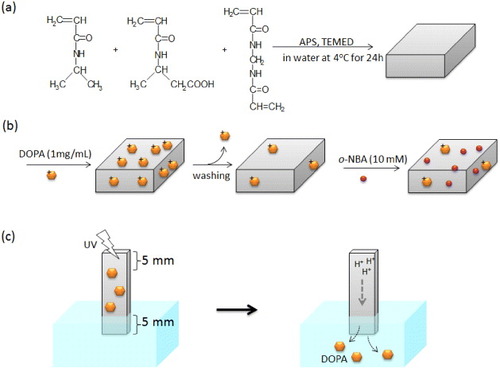
Figure 2 (a) Effect of various NBA derivatives on the proton releasing kinetics in water under UV illumination (10 mM, 19.90 mW cm−2). (b) Effect of UV intensity on the proton releasing kinetics of o-NBA in water.
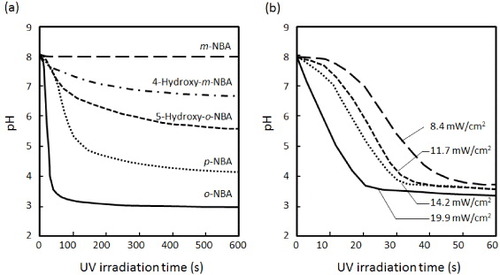
Figure 3 Time-dependent color changes of o-NBA-integrated P(NIPAAm-co-CIPAAm) gels after UV irradiation. The gels were swollen in pH 7 solution containing 10 mM of o-NBA and 1 mg ml−1 of bromocresol green as a pH-indicator (yellow: < pH 3.8, blue: > pH 5.4). The left half of the sample was exposed to UV light through a photomask for 10 s (a), 60 s (b) and 180 s (c) (bar = 5 mm).
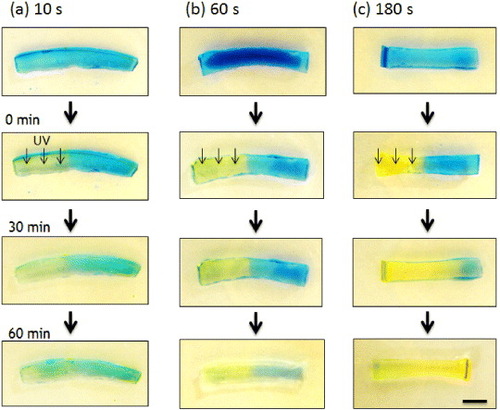
Figure 4 Time-dependent profiles of proton concentrations inside the hydrogels calculated by a simple 1D model. The initial proton concentrations were calculated from the proton release kinetics (figure (b)) to be 3.2 × 10−6 M (a), 3.2 × 10−4 M (b) and 1.0 × 10−3 M (c) for 10, 60 and 180 s UV irradiation, respectively.
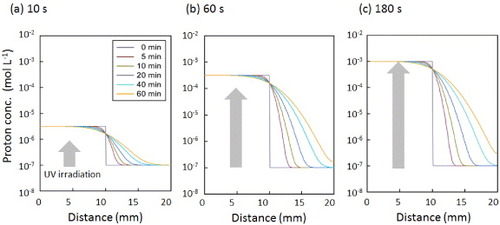
Figure 5 Distributions of pH within the gel calculated by a simple 1D model. The iso-pH points are shown in different colors: yellow < pH 3.8 < green < pH 5.4 < blue. The initial proton concentrations were calculated from the proton release kinetics (figure (b)) to be 3.2 × 10−6 (a), 3.2 × 10−4 (b) and 1.0 × 10−3 M (c) for 10, 60, and 180 s UV irradiation, respectively.
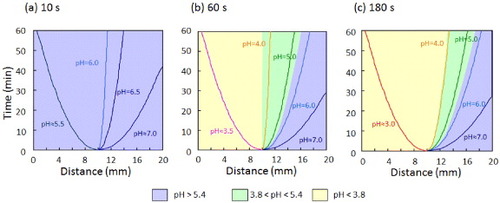
Figure 6 Drug release profiles of DOPA from o-NBA-integrated P(NIPAAm-co-CIPAAm) gels with (solid circles) and without (open circles) UV irradiation for 180 s. The experimental setup is illustrated in scheme (c).
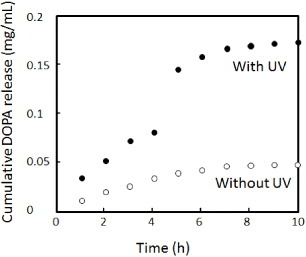
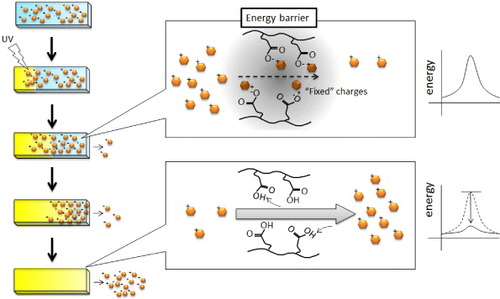
Figure 7 Schematic of the release mechanism of a positively charged drug from negatively charged hydrogels. Before UV irradiation (neutral pH), CIPAAm could stably interact with DOPA because the –COOH groups were more negatively charged above the pKa of CIPAAm above. After UV irradiation (acidic pH), the electrostatic interaction between CIPAAm and DOPA weakened because the –COOH groups became more protonated. The permeation of the dissociated DOPA, however, was still reduced by the electrostatic energy barrier. Therefore, the explosive release of DOPA was observed when the energy barrier completely disappeared after the boundary of pKa reached the non-irradiated end of the gel.