Figures & data
Figure 1. Schematic of cell adhesion. Cell adhesion is a complex process that can be divided into different phases. A floating cell (1) can physically contact with the substrate. In the first phase of cell–matrix interaction (2) the cell adheres passively and starts sensing the substrate. This phase is guided by gravity and followed by cell reshaping and the concomitant adhesion processes (3), resulting in an immature form of the focal adhesions (4). These structures are macromolecular complexes connecting the extracellular matrix to the cytoskeleton, the scaffold of the cell. A number of docking and shuttling proteins are involved in such a process.
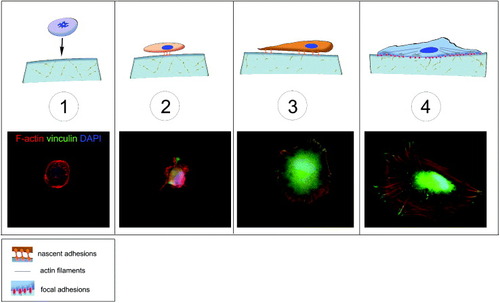
Figure 2. Structure of mature focal adhesions. Mature focal adhesions are macromolecular complexes composed of a number of proteins bridging between the extracellular matrix (ECM) and the cytoskeleton. The mechanical signals arising from the ECM are transduced via the integrins and a number of docking proteins (vinculin, talin, paxillin, zyxin etc) to the actin filaments. Myosin II is involved in cell tension sensing. Some of the proteins found in the focal adhesions can shuttle to the nucleus to act as co-activators of gene transcription. A prominent role in integrin signaling is played by focal adhesion kinase (FAK), a protein tyrosine kinase recruited at an early stage of focal adhesion formation and directly involved in the recruitment of proteins containing SH-2 and SH-3 domains.
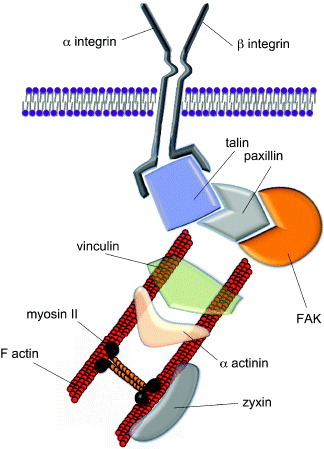
Figure 3. Cell adhesion rates of (a) hMSCs and (b) NHDFs assessed by AlamarBlue® assay. Cells were seeded onto PCL of increasing stiffness (0.9, 1.5, 50 and 133 MPa) and TCPS. Cells grown on low-adhesion plates were used as control (suspension). Fluorescent intensity of each sample was measured after 3 and 8 h of culture at F544/F590. Readings were normalized to the background intensity and presented as percentage change of TCPS after 3 h of culture.
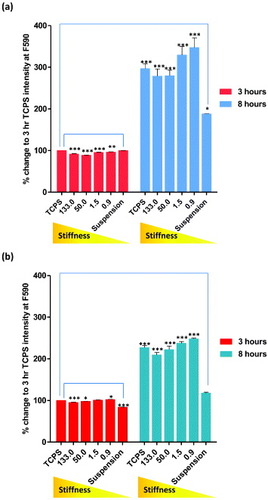
Figure 4. Human mesenchymal stem cells do not express vinculin protein when grown on a very compliant substrate. The cells were grown on growth-factor-reduced MATRIGEL™ for 8 h (upper line) and 24 h (central line) and stained for F-actin (red) and vinculin (green). Nuclei are counterstained with 4',6-diamidino-2-phenylindole (DAPI). The formation of tubules within the 3D microenvironment is accompanied by clear cytoskeletal rearrangement and morphology change, while vinculin expression could not be detected, as shown by higher-magnification images (bottom row).
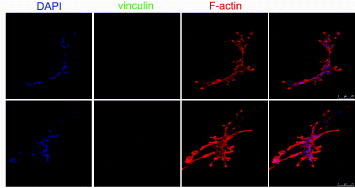
Figure 5. Localization and maturation of focal adhesions, actin cytoskeleton in hMSCs and NHDFs cultured on PCL. Confocal fluorescence micrographs of F-actin (phalloidin staining), vinculin, and nuclei (DAPI) for (a) hMSC and (b) NHDF, 8 and 24 h after seeding. White arrows indicate the formation of vinculin-rich spikes. Representative immunofluorescence images of single hMSC plated on PCL for 24 h, showing (c) co-localization of talin-GFP with paxillin and counterstained with DAPI, and (d) zyxin with vinculin. Scale bars: 25 μm.
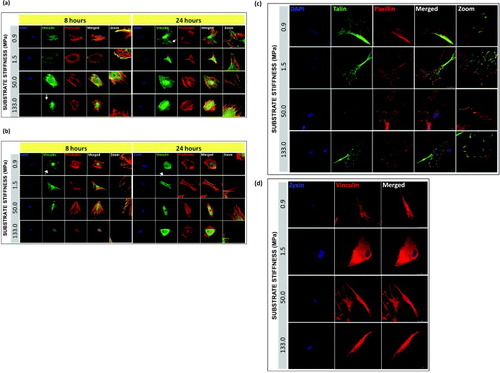
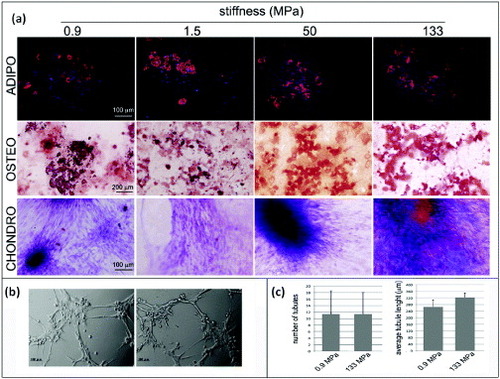
Figure 6. Human mesenchymal stem cell multipotency on PCL films with different stiffnesses. hMSCs were cultured on PCL films and switched to specific differentiation media for 14 days. The formation of lipid vacuoles (FABP4 staining, red), calcium deposits (ALIZARIN RED S staining, red) is shown in cells cultured in adipogenic (ADIPO) or osteogenic media (OSTEO). The occurrence of chondrogenic (CHONDRO) differentiation after 14 days in differentiation medium was confirmed by Masson's trichrome staining. (a) hMSCs grown for 1 week on the softest (0.9 MPa, left) or the stiffest (133 MPa, right) substrates were detached and seeded onto growth-factor-reduced MATRIGEL™ for 3 h. (b) Formation of tubular structures (Scale bars: 25 μm). (c) Number of tubules and the average tubule length measured by ImageJ software.