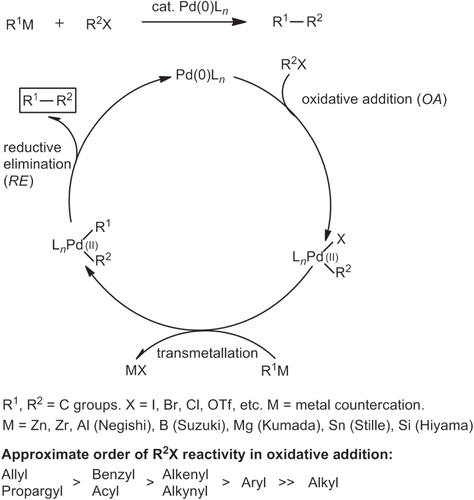
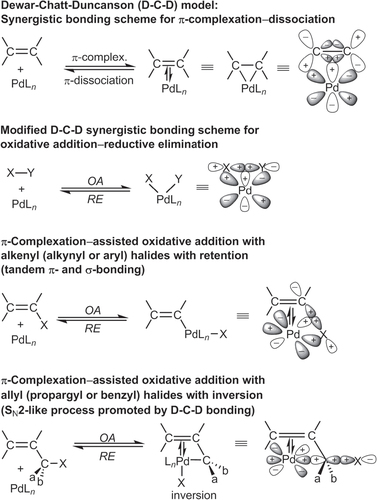
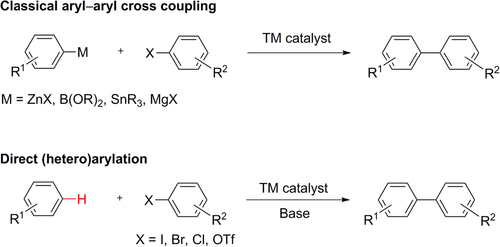
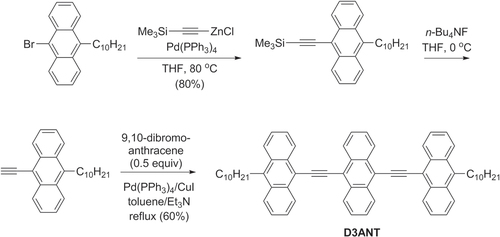
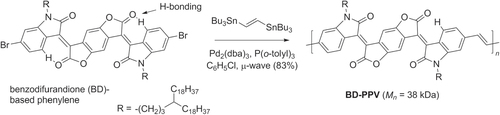
Figures & data
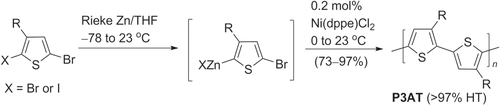
Scheme 3. Regioregular synthesis of head-to-tail poly(3-alkylthiophenes) (HT-P3ATs) using Ni-catalyzed Negishi conditions: dppe = 1,2-bis(diphenylphosphino)ethane.
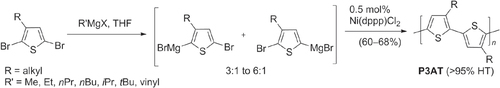
Scheme 4. Regioregular HT-P3ATs prepared using Ni-catalyzed Kumada conditions: dppp = 1,2-bis(diphenylphosphino)propane.
Scheme 6. P3AT synthesis using modified Suzuki conditions (bpy = bipyridine; COD = 1,5-cyclooctadiene; Pin = pinacolato; S-Phos = P(cyclohexyl)2(2,6-(MeO)2biphenyl); Th = 2-thienyl).
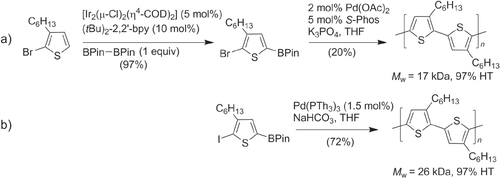
Scheme 8. Synthesis of conductive polythiophenes with TT bithiophene subunits (PQT, PBT−TT, PBT−DTP) using Stille copolymerization: dba = dibenzylideneacetone; DMF = dimethylformamide.
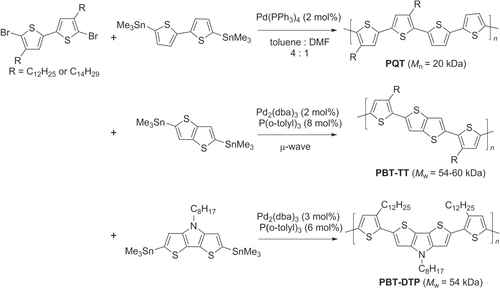
Scheme 9. Comparison of Pd-catalyzed polymerization with direct C−H bond activation versus Stille coupling in the synthesis of PTPD-BT.
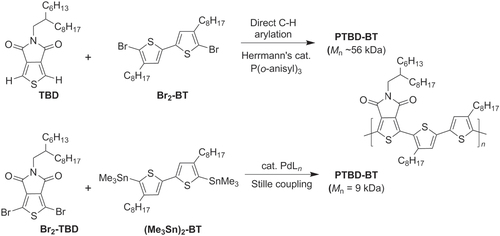
Scheme 10. Phenanthroline-bridged oligothiophenes prepared by Negishi cross-coupling. NIS = N-iodosuccinimide.
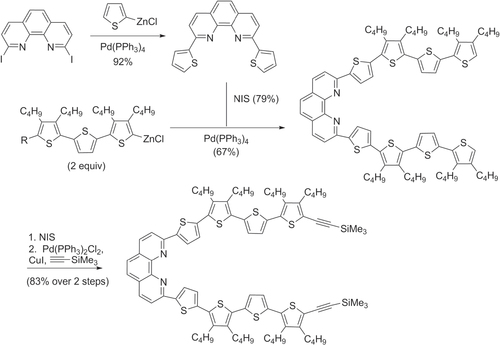
Scheme 11. Benzothiadiazole−cyclopentadithiophene (BTZ-CDT) copolymers with exceptionally high hole mobilities, prepared by Suzuki polycondensation. Aliquat 336 = trioctyl-methylammonium chloride; Pin = pinacolato.
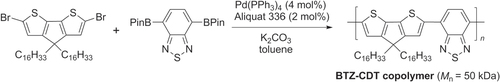
Scheme 13. Homopolymerization of AA-type monomer into a poly(9,9-bis(3,6-dioxaheptyl)-fluorene-2,7-diyl) by Ni-catalyzed Yamamoto coupling. bipy = 2,2′-bipyridine; DMF = dimethylformamide.
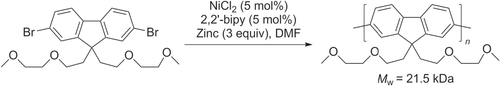
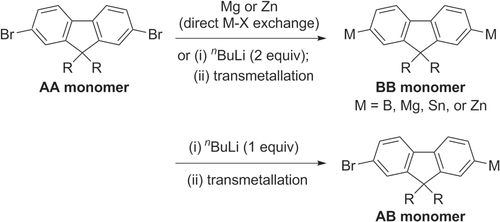
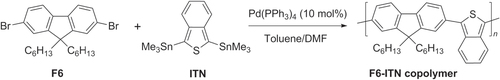
Scheme 14. Heteropolymerization of AA- and BB-type monomer into poly(9,9-dihexyl)-fluorene by Suzuki condensation under conventional or microwave-assisted heating conditions. dppf = 1,1′-bis(diphenylphosphino)ferrocene; Pin = pinacolato.

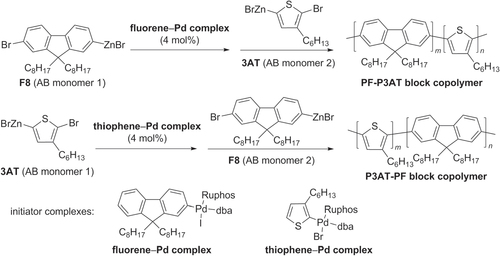
Scheme 15. Homopolymerization using Negishi coupling conditions with an air-stable fluorene−Pd complex: dba = dibenzylideneacetone; Ruphos = 2′,6′-bis(1-methylethoxy)(1,1′-biphenyl)-2-yl)dicyclohexylphosphine.
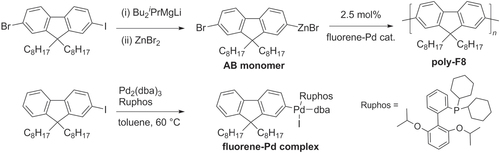
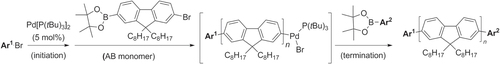
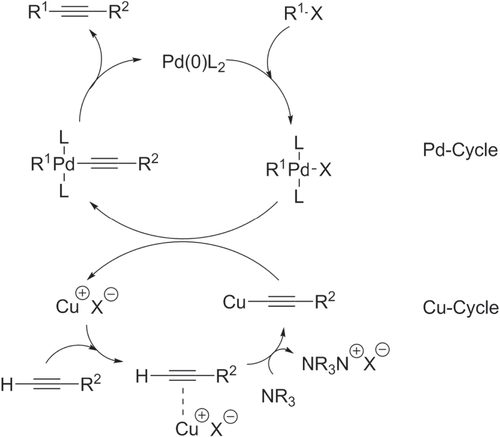
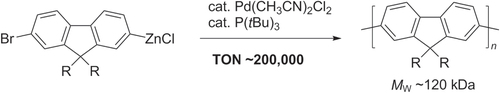
Scheme 16. Pd/PtBu3-catalyzed Negishi chain-growth polycondensation of AB-type monomers proceeds with exceptionally high catalytic efficiency.
Scheme 18. Hybrid copolymer with alternating DTB and fluorene units, prepared by Suzuki polycondensation.
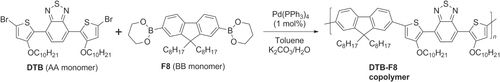
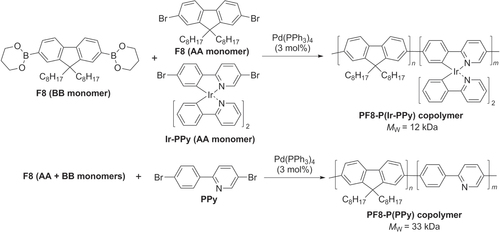
Scheme 19. Electroluminescent dioctylfluorene−benzothiadiazole (F8−BT) copolymer, prepared by dispersion polymerization using Suzuki conditions.
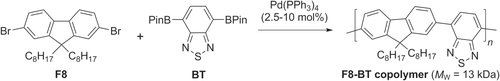
Scheme 20. Top, synthesis of helical tetrathiafulvalene−fluorene (TTFV-F) copolymers by Sonogashira coupling. Bottom, helical conformation of TTFV-F, before and after complexation with SWCNT [Citation84]. Reprinted with permission from the American Chemical Society.
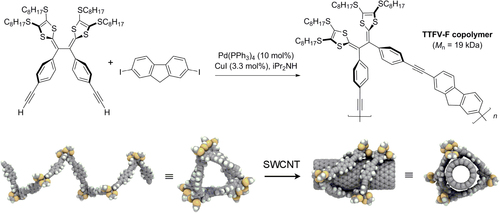
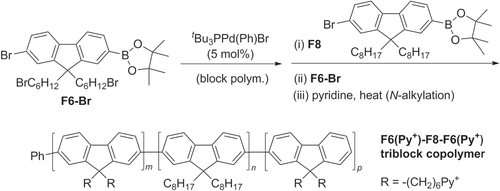
Scheme 22. Synthesis of diblock π-conjugated PF−P3AT copolymers based on a Negishi coupling protocol, by sequential addition of AB-type monomers: dba = dibenzylideneacetone; Ruphos = 2′,6′-bis(1-methylethoxy)(1,1′-biphenyl)-2-yl)dicyclohexylphosphine.
Scheme 25. Synthesis of protohelical poly(3-butoxy-4′,5-meta-biphenylene (PBmP) by Suzuki polycondensation.
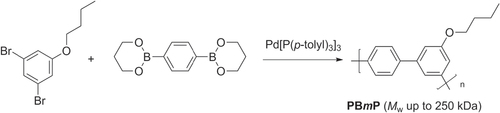
Scheme 27. Improvement in chain-growth polymerizations based on Kumada cross-coupling, using electron-rich diphosphine ligands.
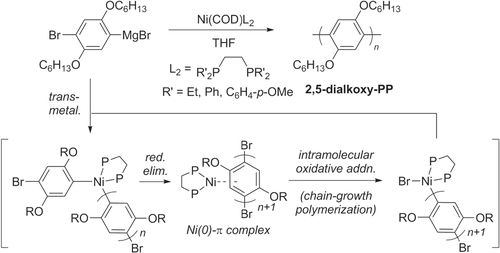
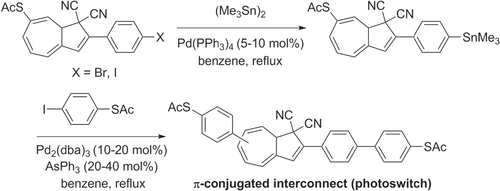
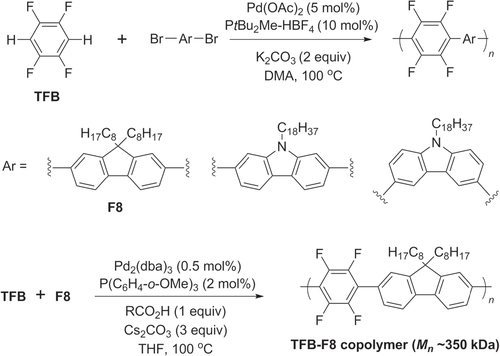
Scheme 28. Pd-catalyzed C−H activation and polycondensation of tetrafluorobenzene (TFB) into heteroarylene copolymers. Ac = acetyl; dba = dibenzylideneacetone; DMA = dimethylacetamide.
Scheme 29. Direct C−H activation in the synthesis of π-conjugated diketopyrrolopyrroles (DPPs). DMA = dimethylacetamide; Piv = pivaloyl.
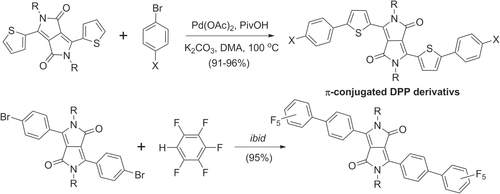
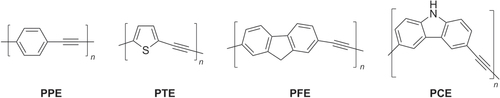
Figure 4. Structures of representative PAEs. PPE = poly(phenylene ethynylene), PTE = poly(thienylene ethynylene), PFE = poly(fluorene ethynylene), PCE = poly(carbazoyl ethynylene).
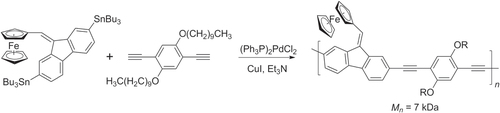
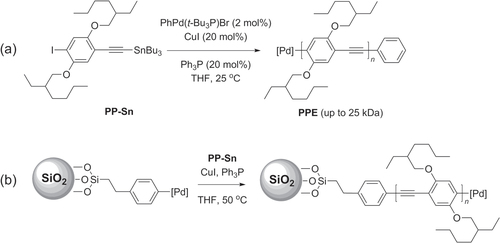
Scheme 31. (a) Synthesis of PPEs with controlled polydispersity by catalytic transfer polycondensation (CTP) using Sonogashira-like conditions. (b) Surface-initiated polymerization of PPE under similar conditions.
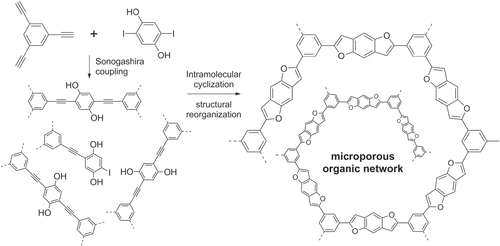
Scheme 32. Synthesis of a benzodifuran-containing microporous organic network (MON) from a hyperbranched PPE precursor.
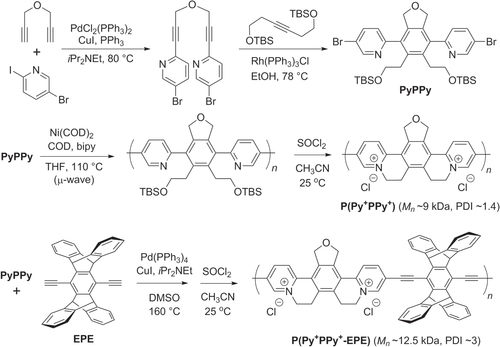
Scheme 33. Synthesis of redox-active copolymers having rigid, π-conjugated backbones, using Yamamoto and Sonogashira coupling conditions. Bipy = 2,2′-bipyridine; COD = cyclooctadiene; EPE = ethynyl(pentiptycenyl)ethynylene; Py+PPy+ = pyridinium(phenyl)pyridinium; TBS = t-butyldimethylsilyl.
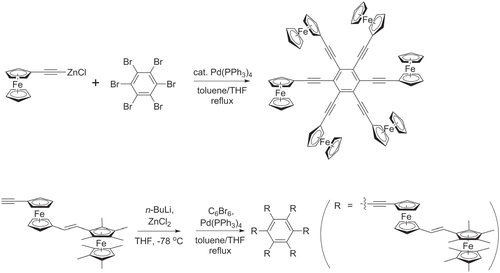
Scheme 34. One-pot syntheses of hexakis(ferrocenylethynyl)benzene and hexakis((E)-(2-(ethynylferrocenyl)ethynyl)octamethylferrocene)benzene, using multiple Negishi couplings.
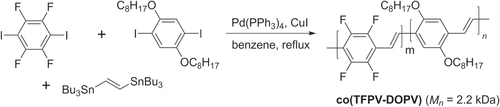
Scheme 37. Synthesis of tetrafluorophenylvinyl−dialkoxyphenylvinyl copolymer (co(TFPV-DOPV)) using Stille conditions.
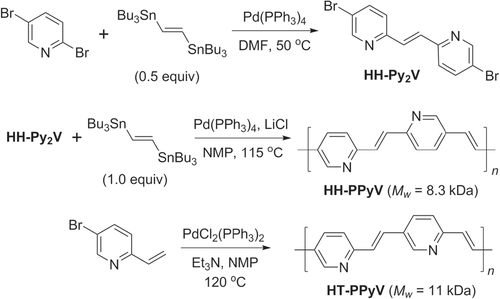
Scheme 38. Synthesis of regioregular poly(methylpyridinium vinylene)s (HH- and HT-PMPyVs) by Stille and Heck couplings, respectively. NMP = N-methylpyrrolidinone.
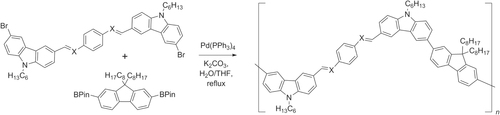
Scheme 40. Synthesis of PAV copolymers and their poly(azomethine) analogs by Suzuki polycondensation. Pin = pinacolato.
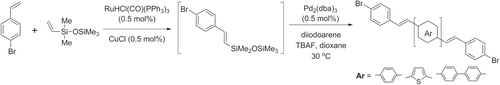
Scheme 41. One-pot tandem synthesis of oligophenylvinylenes from 4-bromostyrene, using olefin cross-metathesis and Hiyama coupling; dba = dibenzylideneacetone; TBAF = tetrabutylammonium fluoride.
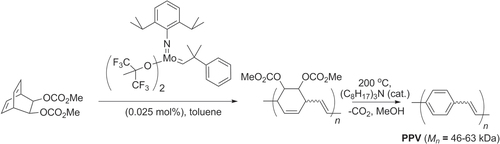
Scheme 42. PPV synthesis by ROMP of a disubstituted bicyclooctadiene, followed by thermal elimination.