Figures & data
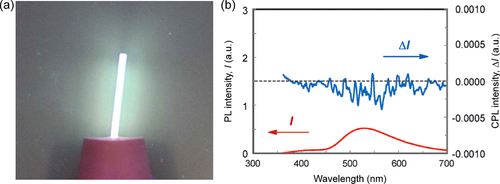
Figure 1. (a) A photograph of a quartz plate coated with P1 film and illuminated by UV light (λ = 365 nm). (b) Photoluminescence (PL) and circularly polarized luminescence (CPL) spectra of P1 film annealed at 120 °C for 6 h. Adapted with permission from ref. [Citation60]. Copyright 2011 The Royal Society of Chemistry.
Scheme 2. Lyotropic liquid crystalline poly(phenylacetylene) derivatives with hydrophilic (P3) or lipophilic side chains (P4).
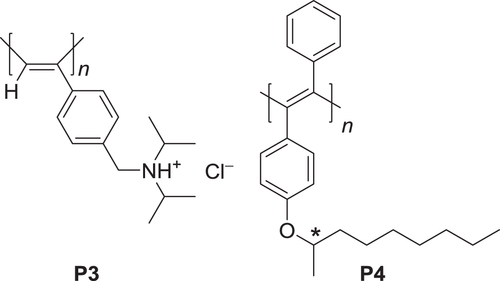
Figure 2. (a) Circular dichroism (CD) spectra of P3 with (R)- or (S)-NaPLA in water and UV−vis absorption spectrum of P3 with (R)-NaPLA in water. The concentration of P3 is 4.0 × 10−3 m. The mixing ratio is [NaPLA]/[P3] = 0.5. (b) Schematic illustration of chiral amplification in macromolecular helicity in dilute solution and liquid crystal (LC) state. P3 has interconvertible, right- (green chain) and left-handed (blue chain) helical conformations. In dilute solution, a small amount of chiral acid (NaPLA) induces excess of one-handed helical sense on the P3 helix. In lyotropic LC state, the induced chirality is significantly amplified, resulting in a one-handed helical supramolecular assembly. Adapted with permission from ref. [Citation63]. Copyright 2006 American Chemical Society.
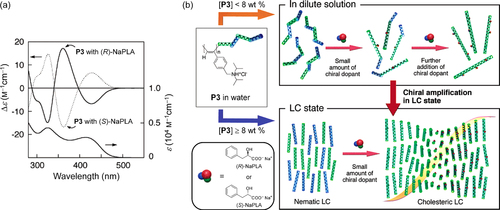
Figure 3. (a) Polarized optical microscopy (POM) image of the lyotropic chiral nematic LC (N*-LC) phase of (S)-P4 in toluene (c = 10 wt%) showing a double-spiraled texture. Inset shows a fingerprint texture with a half helical pitch (p/2) of 1.5 μm. (b) Schematic representation of the N*-LC phase of (S)-P4. The polymers self-assemble into the interchain helical structure. The polymer interchain distance in an N*-LC domain is described in the top view. Adapted with permission from ref. [Citation64]. Copyright 2012 American Chemical Society.
Scheme 3. Thienylene- and phenylene-based aromatic conjugated polymers (ACPs) with chiral nonyl substituents.
Figure 4. POM images of (R)-P6 at (a) 200 °C and (b) 150 °C and of (c) (R)-P7 at 156 °C during the cooling process. Reprinted with permission from ref. [Citation65]. Copyright 2012 American Chemical Society.
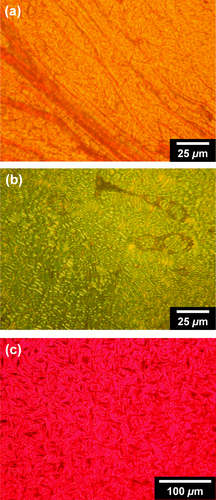
Figure 5. UV−vis absorption (upper) and CD (lower) spectra of polymers in chloroform (c = 1.0 × 10−4 m) for (a) P5s, (b) P6s, and (c) P7s and the as-cast films of (d) P5s, (e) P6s, and (f) P7s. The solid and dotted lines indicate the spectra of polymers with (R)- and (S)-configurations, respectively. Reprinted with permission from ref. [Citation65]. Copyright 2012 American Chemical Society.
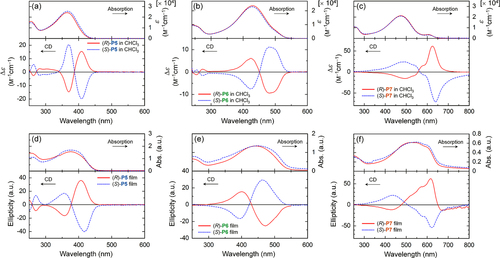
Figure 6. Schematic of the interchain helically π-stacked structure of P7 assembly. Reprinted with permission from ref. [Citation65]. Copyright 2012 American Chemical Society.
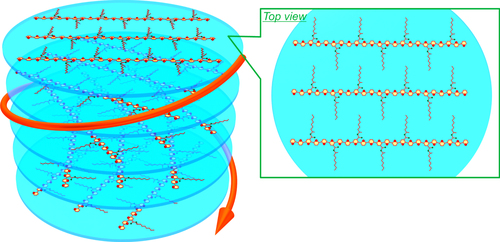
Figure 7. PL (upper), CPL (middle), and luminescence dissymmetrty factor glum (lower) spectra of the polymers in (a) chloroform (c = 1.0 × 10−4 m) and (b) as-cast films. (c) PL (upper), CPL (middle), and glum (lower) spectra of P7 in chloroform (c = 1.0 × 10−4 m), the as-cast films, and the annealed films. Reprinted with permission from ref. [Citation65]. Copyright 2012 American Chemical Society.
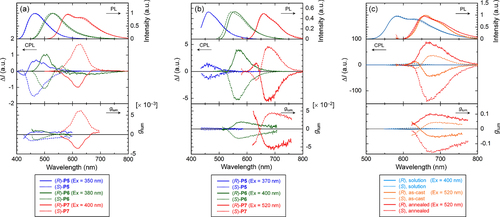
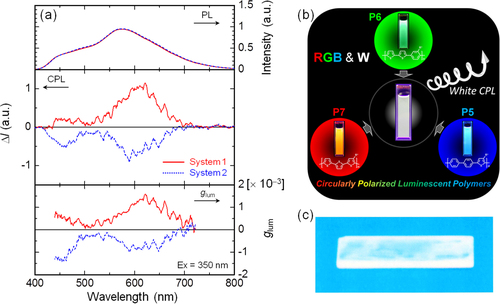
Figure 8. (a) PL (upper), CPL (middle), and glum (lower) spectra of the polymer mixtures in chloroform [P5, 5.0 × 10−5 m; P6, 5.0 × 10−5 m; P7, 2.5 × 10−4 m], where system 1 is the mixture of (R)-P5, (R)-P6, and (S)-P7, and system 2 is a mixture of (S)-P5, (S)-P6, and (R)-P7. (b) RGB and white fluorescence of system 1, the mixture of (R)-P5, (R)-P6, and (S)-P7, in chloroform and (c) white fluorescence of the cast film of system 1 on a quartz substrate. The film was prepared by dissolving the mixture and an excess of polystyrene in chloroform followed by casting onto the quartz substrate. UV light at 365 nm was used for excitation. Reprinted with permission from ref. [Citation65]. Copyright 2012 American Chemical Society.
Scheme 4. Conjugated polymer (P8) and molecules (9 and 10) exhibiting external stimuli-responsive CPL.
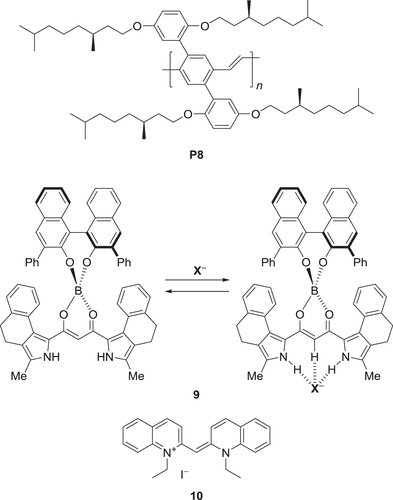
Figure 9. (a) The dissymmetry factors in absorption (lines) and luminescence (markers), and (b) normalized absorption (solid lines) and luminescence (dashed lines) spectra of P8 solutions in DCE, CHCl3, and CHCl3−MeCN (50:50). Adapted with permission from ref. [Citation71]. Copyright 2006 American Chemical Society.
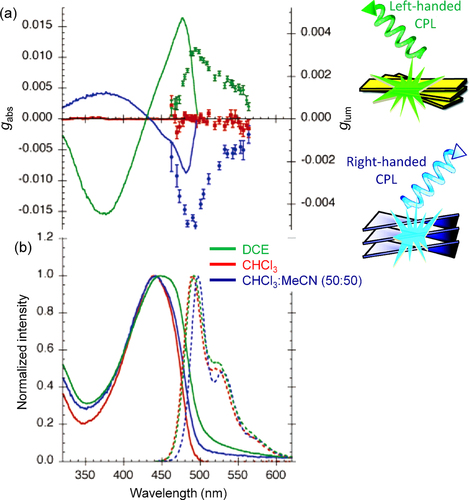
Figure 10. Spectral changes for 9 (1.0 × 10−5 m in CH2Cl2) in (a) UV−vis absorption (bottom) and CD (top) and (b) fluorescence (bottom) and CPL (top) measurements upon the addition of Cl− as the TBA salt (50 equiv for UV−vis absorption and 200 equiv for the other measurements; 9: black line; 9·Cl−: red line), and photographs of the corresponding solutions (insets). Reprinted with permission from ref. [Citation73]. Copyright 2011 American Chemical Society.
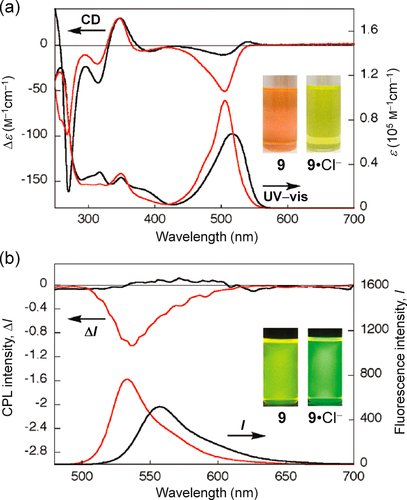
Figure 11. (a) CD (blue) and CPL (red) spectra, and (b) UV−vis absorption (blue) and PL (red) spectra of the induced J-aggregates of 10 (c = 3 × 10−5 m) with (+)-Rochelle salt (c = 1.8 m) in aqueous solution at 5 °C. Excitation wavelength: 470 nm. Reprinted with permission from ref. [Citation73]. Copyright 2012 The Chemical Society of Japan.
Scheme 5. Structures of (i) a zinc porphyrin dendrimer 11 and (ii) an oligomeric host gelator 12 and a guest luminophore, rhodamine B.
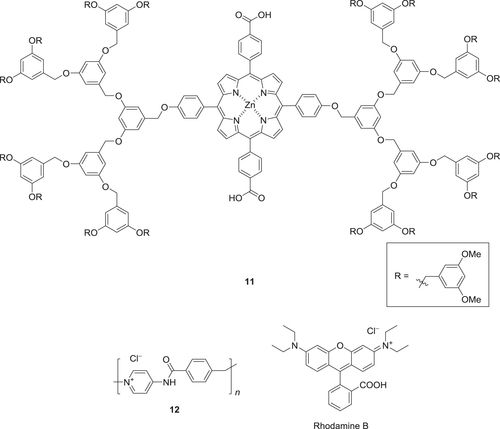
Figure 12. (a) UV−vis absorption and (b) CD spectra of a benzene solution of 11 (c = 6.0 × 10−6 m) in a 10 × 10 × 40 mm3 quartz optical cell. The CD spectra were measured upon bottom rotary stirring at 1350 rpm in clockwise (CW; blue) and counterclockwise (CCW; red), and without stirring (OFF; black). (c) Change in CD intensity at 453 nm in response to a stepwise variation of the stirring conditions at 20 °C. (d) A transmission electron micrograph of air-dried sample of 11 benzene solution on a specimen grid. Scale bar: 500 nm. Adapted with permission from ref. [Citation74]. Copyright 2007 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
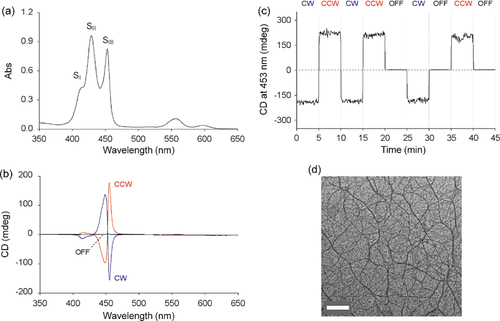
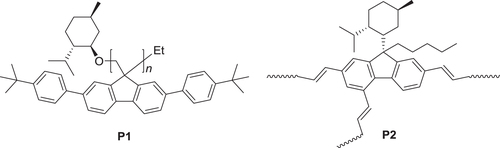
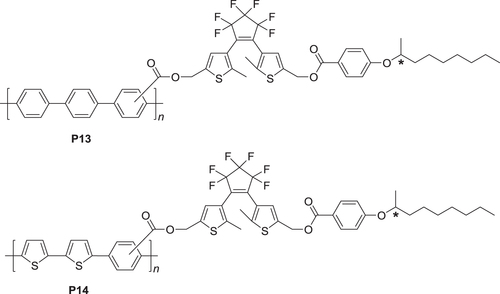
Figure 13. UV−vis absorption spectra and difference spectra between the PSS and the open state of (a, b) (R)-P13 and (d, e) (R)-P14 cast films, respectively, and CD spectra of (c) P13 and (f) P14 cast films in the open state and the PSS of the dithienylethene moiety. Reprinted with permission from ref. [Citation47]. Copyright 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
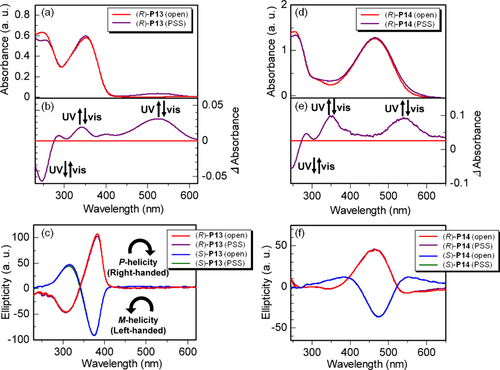
Figure 14. PL spectra of (a) (R)-P13 and (c) (R)-P14 cast films and CPL spectra of (b) P13 and (d) P14 cast films. The wavelength of excitation light was 350 nm for P13 and 380 nm for P14. Insets show photographs of (R)-P13 and (R)-P14 films in the open state and PSS under UV irradiation (excitation wavelength = 370 nm, 4 W, handheld lamp). Reprinted with permission from ref. [Citation47]. Copyright 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
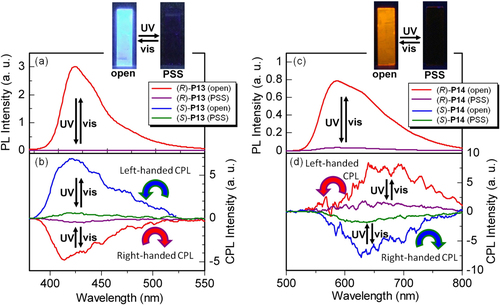
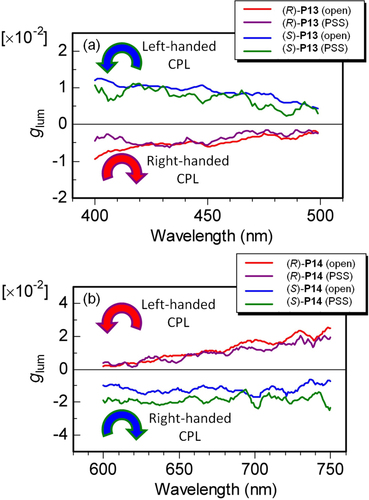
Figure 15. Luminescence dissymmetry factors (glum) of (a) P13 and (b) P14 films in open state and PSS. Reprinted with permission from ref. [Citation47]. Copyright 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Scheme 7. An achiral cationic polythiophene derivative (P15) and chiral polysaccharides (SPG and Cur-oeg).
Figure 16. (a) Schematic illustrations of the supramolecular helical nanowire formation in solution. (b) CD (dashed line) and CPL (solid line), and (c) UV−vis absorption (dashed line) and fluorescence (solid line) spectra of P15−SPG complex in water−DMSO. Excitation wavelength: 400 nm. Adapted with permission from ref. [Citation77]. Copyright 2009 The Chemical Society of Japan.
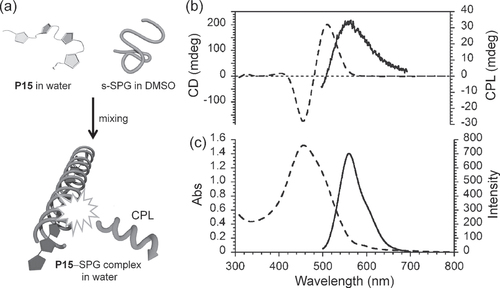
Figure 17. (a) Photographs and (b) UV−vis absorption spectra of a P15−Cur-oeg complex film. The as-prepared film was exposed to humid air and methanol vapor, and then dried in vacuo again. Reprinted with permission from ref. [Citation79]. Copyright 2010 American Chemical Society.
Scheme 8. An achiral cationic poly(para-phenylene) derivative (P16) and axially-chiral anionic binaphthyl derivatives (BNPs) with (R)- and (S)-configurations.
Figure 18. (a) The UV−vis absorption, CD, and absorption dissymmetry factor (gabs) spectra and (b) PL, CPL, and glum spectra of P16, BNP, and a mixture (P16−BNP) (1.0:2.0 mol/ mol) in methanol−water (50:50). The concentrations are as follows: P16, 5.8 × 10−5 m; BNP, 2.0 × 10−5 m; P16 in the mixture, 2.0 × 10−5 m. (c) The UV−vis absorption (upper) and CD (lower) spectra of P16−( R )-BNP in methanol−water (50:50) at various molar ratios. The concentration of P16 is held constant at 2.0 × 10−5 m. (d) The UV−vis absorption, CD, and gabs spectra of P16−( R )-BNP (1.0:2.0 mol/ mol) in methanol−water (50:50 v v−1) at various temperatures during the heating process. The concentration of P16 is 2.0 × 10−5 m. Adapted with permission from ref. [Citation30]. Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
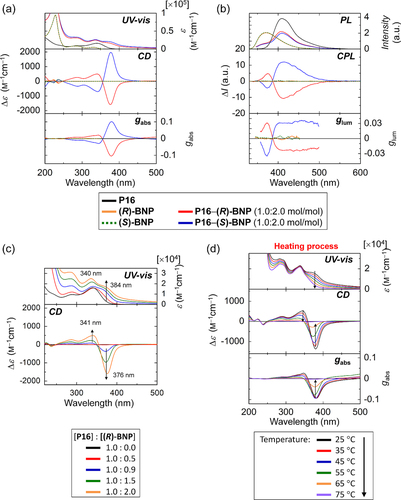
Figure 19. Scanning electron microscopy images of P16−BNP (1.0:2.0 mol/ mol) prepared by (a) drying the mixed solution of P16 and BNP at room temperature and (b) freeze-drying the mixed solution. (c) A fluorescence microscopy image of P16−BNP (1.0:2.0 mol/ mol) spin-coated on a glass plate. Fluorescence was excited by UV light (λ = 365 nm) and a low-pass filter was employed to remove wavelengths shorter than 420 nm. (d) POM image of P16−BNP (1.0:2.0 mol/ mol) in methanol−water (50:50) at room temperature. The crossed arrows represent the directions of the polarizer and the analyzer of polarized optical microscope. Reprinted with permission from ref. [Citation30]. Copyright 2012 WILEY-VCH Verlag GmbH & co. KGaA, Weinheim.
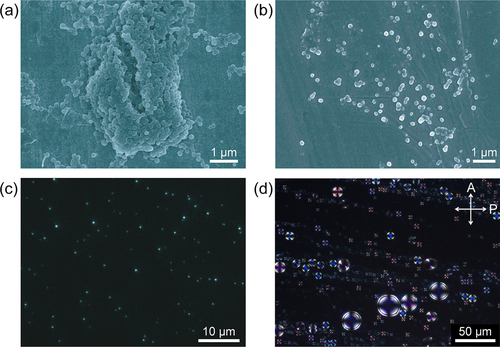
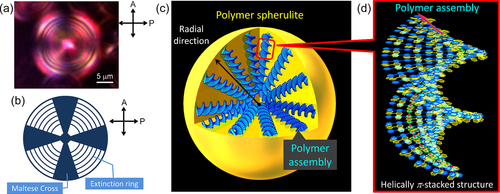
Figure 20. (a) A Maltese Cross image with the extinction rings observed in POM for a spherulite of P16−( R )-BNP (1.0:2.0 mol/ mol) in methanol−water (50:50) at room temperature. (b) The schematic pattern of the Maltese Cross image with extinction rings. (c) A plausible model of a spherulite consisting of polymer assemblies. (d) The plausible model of polymer assembly with the helically π-stacked structure. P16 and BNP are represented in blue and yellow, respectively. Reprinted with permission from ref. [Citation30]. Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.