Figures & data
Polymerisation conditions of PP samples.
Polymerisation conditions of EP copolymers.
Polymerisation conditions of PE samples.
Viscosimetry, DSC and 13C-NMR characterisation of PP samples.
Viscosimetry, DSC and 13C − NMR characterisation of EP copolymers.
Viscosimetry and DSC characterisation of PE samples.
Figure 1 Mechanodynamical loss modulus curve of PP92.4. (○) Experimental, (—) calculated, (····) single components.
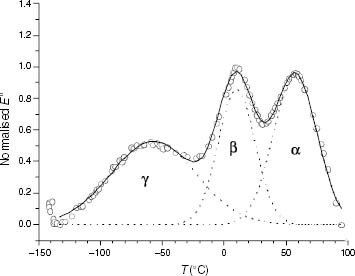
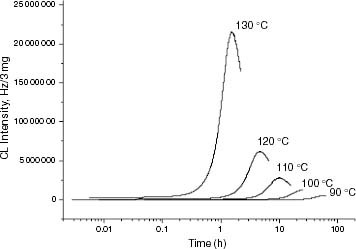
Figure 4 ICL kinetics of iPP samples at 120 °C: (○) PP178.1, (—) PP147.8, (····) PP131.9, (– – –) PP99.5, (–·–) PP92.4, (□) PP77.9.
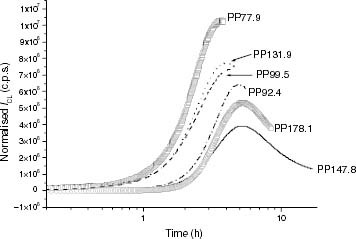
Figure 5 ICL kinetics of propylene-rich EP samples at 120 °C: (—–) EP0.6, (– – –) EP1.1, (····) EP3.3, (–·–) EP5.1, (—) EP6.1, (–··–) EP23.3.
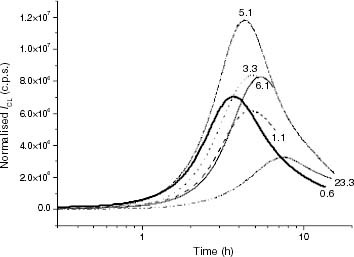
Figure 6 ICL kinetics of PE and ethylene-rich EP samples at 120 °C: (—–) PE242.5, (—) EP99.7, (– – –) EP99.0.
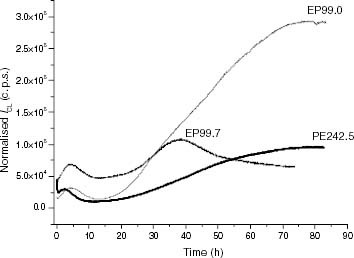
Figure 7 Photoluminescence emission spectra: (a) PP and propylene-rich EP samples; (b) PE and ethylene-rich EP samples obtained under irradiation at λ exc = 270 nm and T = - 130 °C after a 0.5 s delay. Samples are identified in the graphs.
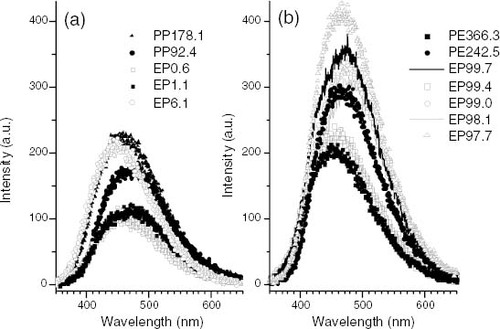
Figure 8 Variation of ti with the molar mass for PP samples (black symbols) and propylene-rich EP copolymers (white symbols).
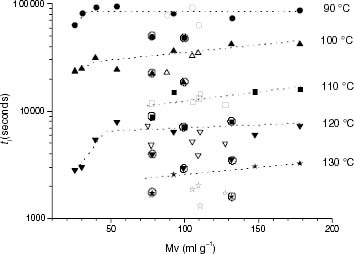
Figure 9 Variation of ti with the content of stereo defects for PP samples (black symbols) and propylene-rich EP copolymers (white symbols).
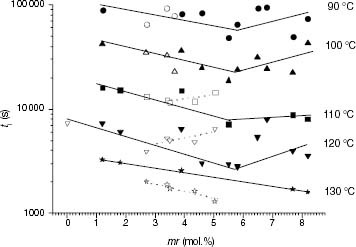
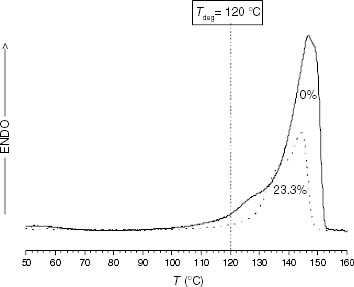
Figure 11 DSC melting endotherms of PP and propylene-rich EP samples (the ethylene content is indicated on each curve).
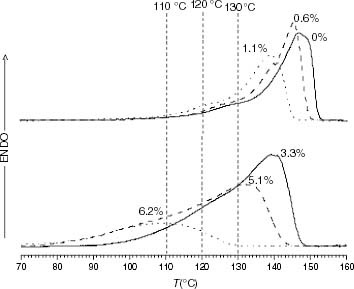
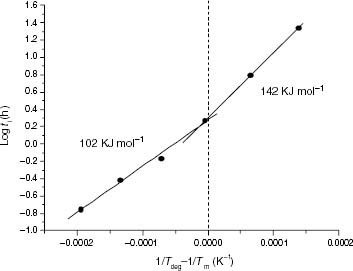
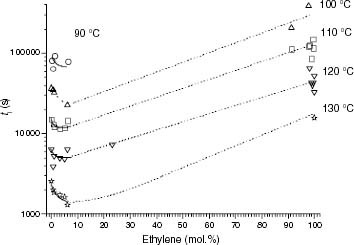
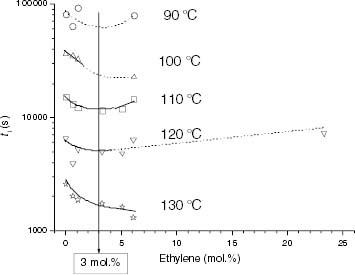
Figure 13 Variation of ti with the ethylene content for the propylene-rich EP samples at 110, 120 and 130 °C. Black points correspond to Tdeg < Tm and red points correspond to Tdeg > Tm.
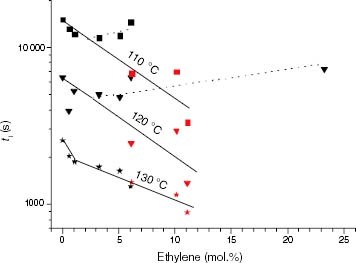
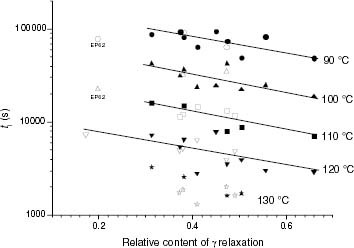
Figure 14 Variation of ti with the total of isotacticity interruptions for PP samples (black symbols) and propylene-rich EP copolymers (white symbols).
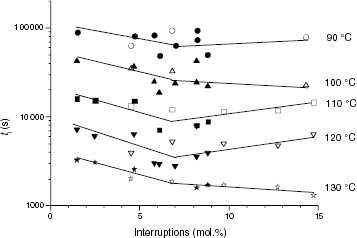
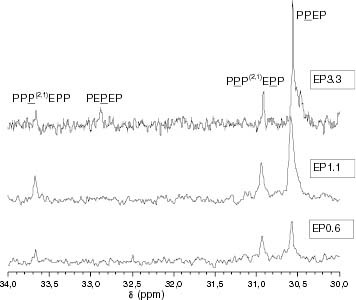
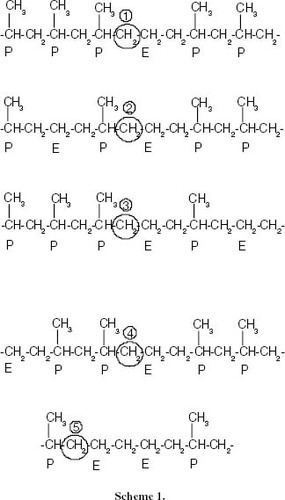
Figure 16 Changes in the 13C-NMR spectrum with the ethylene content showing the region corresponding to propylene-ethylene insertions of methylene, which are contiguous to methines. The chemical environment of the CH2 nuclei is shown in scheme 1.
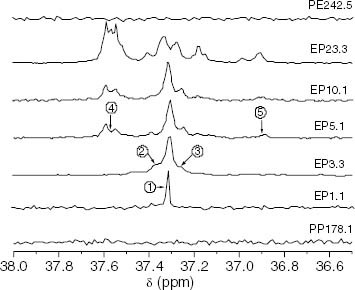
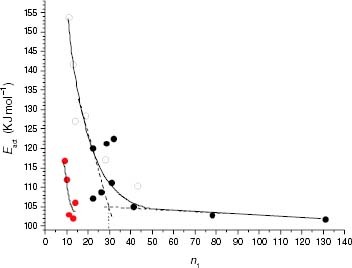
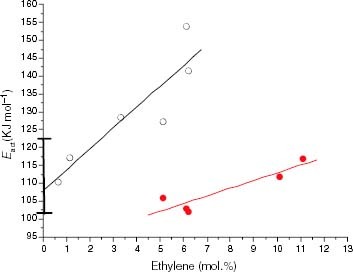
Figure 17 Variation of Eact of the thermo-oxidation in the solid state with the average isotactic length (n1) for PP samples (black symbols) and propylene-rich EP copolymers (white symbols). Red points correspond to the Eact values of EP samples degraded in the melt.