Figures & data
Figure 1 Solid-state cross polarization magic angle spinning (CPMAS) 13C-nuclear magnetic resonance (NMR) spectra of (A) Kawatabi A horizon soil and (B) its extracted humic substance used in the present study. *, Spinning side band.
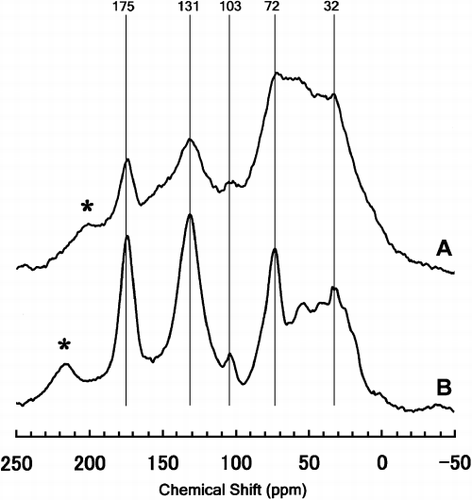
Figure 2 Visible photoabsorption spectra of extracts with NaOH solution from Kawatabi A horizon soil, its extracted humic substance and synthetic Al–humic substance complexes.
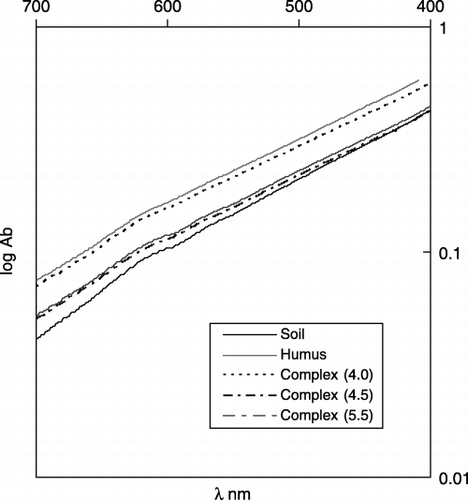
Table 1 Melanic indices (MI) of original Kawatabi A horizon soil, humic substance extracted the soil samples and synthetic Al–humus complexes
Table 2 Total contents of Al and C in synthetic Al–humic substance complexes and extractable Al and C with sodium pyrophosphate and sodium hydroxide-tetraborate
Figure 3 Solid-state magic angle spinning (MAS) 27Al-nuclear magnetic resonance (NMR) spectra of humic substance extracted from Kawatabi A horizon and synthetic Al–humic substance complexes.
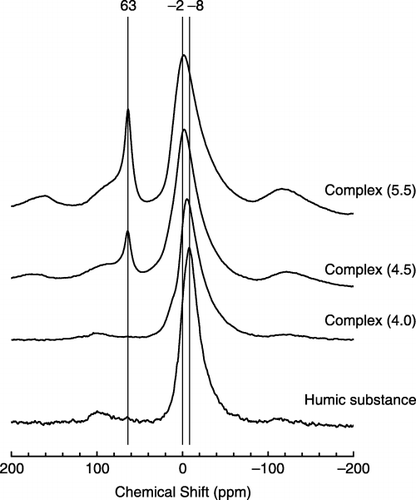
Figure 4 Release rates of Al from synthetic Al–humic substance complexes with 10−3 mol L−1 acetate buffer adjusted to pH 3.5.
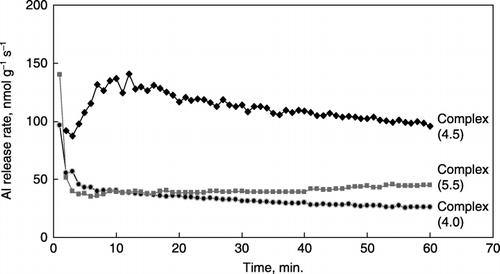
Figure 5 Plot of equilibrium Al solubility versus pH for synthetic Al–humic substance complexes. The solubility of synthetic gibbsite is indicated by the dotted line for comparison. Solubility expressions were pAl = 0.96 pH + 1.09 for Complex (4.0), pAl = 1.43 pH + 1.36 for Complex (4.5), and pAl = 1.49 pH + 1.86 for Complex (5.5).
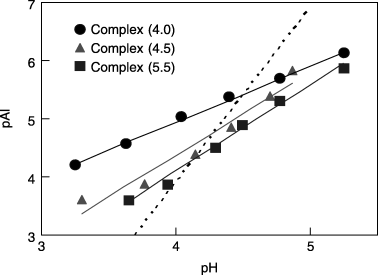
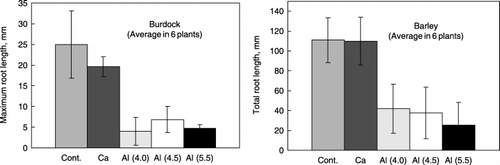
Figure 6 Root growth of burdock and barley cultured in perlite media containing synthetic Al–humic substance complexes (Al(4.0), Al(4.5) and Al(5.5)), Ca–humic substance complex (Ca) and perlite medium only (Cont.). Bars indicate standard deviation.