Figures & data
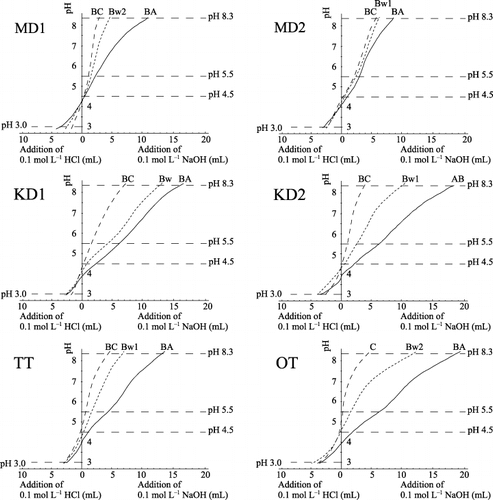
Table 1 Brief description of the soils studied
Table 2 General physicochemical properties of the selected soils
Table 3 Correlation coefficients among the physicochemical properties and titration data of the soils (n = 43)
Figure 2 Relationships between (a) titratable alkalinity and Alo (extraction in the dark with acid [pH 3] 0.2 mol L−1 ammonium oxalate), (b) OH− consumption and exchangeable Al and (c) OH− consumption and pyrophosphate extractable C (Cp).
![Figure 2 Relationships between (a) titratable alkalinity and Alo (extraction in the dark with acid [pH 3] 0.2 mol L−1 ammonium oxalate), (b) OH− consumption and exchangeable Al and (c) OH− consumption and pyrophosphate extractable C (Cp).](/cms/asset/f9c6547d-0c53-4e93-9787-8433811fbff4/tssp_a_10382604_o_f0002g.gif)
Table 4 Soil solution composition
Figure 3 Relationships observed among the soil solution compositions. (a) Anion deficit and dissolved organic carbon (DOC), (b) inorganic monomeric Al (Alinorg) and pH and (c) organic monomeric Al (Alorg) and DOC.
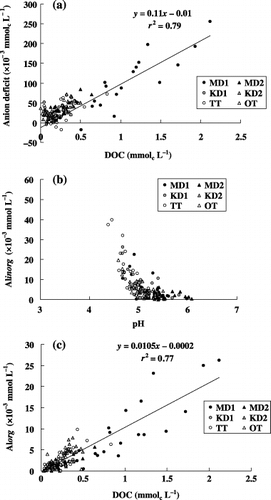
Figure 4 Chemical properties and titration data of the soil profiles superimposed with the soil solution composition. Error bars are standard error. Values with the same letter are not significantly different (P < 0.05). Alo, Feo, extraction in the dark with acid (pH 3) 0.2 mol L−1 ammonium oxalate; Alp, Fep, extraction with 0.1 mol L−1 pyrophosphate at pH 10; DOC, dissolved organic carbon.
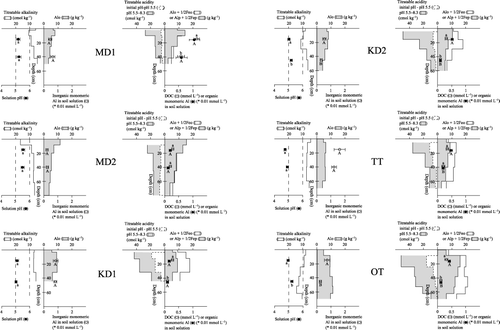
Figure 5 Chemical properties and titration data of the soil profiles in northern Kyoto superimposed with the soil solution composition. Data are cited from Funakawa et al. (1993a). Error bars are standard error. Values with the same letter are not significantly different (P < 0.05). Alo, Feo, extraction in the dark with acid (pH 3) 0.2 mol L−1 ammonium oxalate; Alp, Fep, extraction with 0.1 mol L−1 pyrophosphate at pH 10; DOC, dissolved organic carbon.
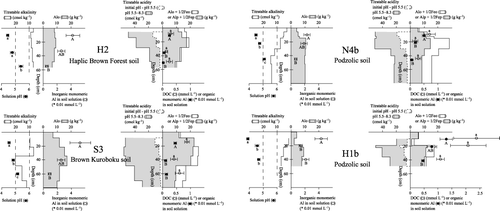
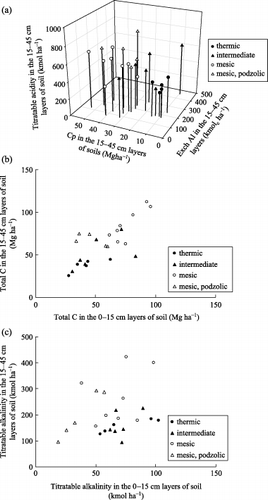
Figure 6 Amounts of (a) titratable acidity, (b) total C and (c) titratable alkalinity on a kmol ha−1 basis. Data presented here include data cited from Funakawa et al. (1993b) and Funakawa et al. (2003). Cp, pyrophosphate extractable C; Exch Al, exchangeable Al.