Figures & data
FIG 1 Maximized z-stack confocal images of the HttQ103 and HttQ103P fragments expressed under different conditions. (a and A) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast following growth overnight in galactose medium. (b and B) HttQ103 and HttQ103P fragments in yeast cured of prions by guanidine following growth overnight in galactose medium. (c and C) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast expressed for 9 h (∼3 generations) in galactose medium. (d and D) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast expressed for 9 h in galactose and guanidine medium. (e and E) HttQ103 and HttQ103P fragments in yeast grown for 6 h with galactose and guanidine, spun, and resuspended in galactose medium for 3 h. (f and F) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast grown overnight at a relatively high density (starting OD of ∼0.4) in galactose medium with guanidine. The insets are an enlargement of the profile drawn through an aggregate. Images of HttQ103 and HttQ103P are shown in panels a to f and A to F, respectively.
![FIG 1 Maximized z-stack confocal images of the HttQ103 and HttQ103P fragments expressed under different conditions. (a and A) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast following growth overnight in galactose medium. (b and B) HttQ103 and HttQ103P fragments in yeast cured of prions by guanidine following growth overnight in galactose medium. (c and C) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast expressed for 9 h (∼3 generations) in galactose medium. (d and D) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast expressed for 9 h in galactose and guanidine medium. (e and E) HttQ103 and HttQ103P fragments in yeast grown for 6 h with galactose and guanidine, spun, and resuspended in galactose medium for 3 h. (f and F) HttQ103 and HttQ103P fragments in [RNQ+] [PSI+] yeast grown overnight at a relatively high density (starting OD of ∼0.4) in galactose medium with guanidine. The insets are an enlargement of the profile drawn through an aggregate. Images of HttQ103 and HttQ103P are shown in panels a to f and A to F, respectively.](/cms/asset/fee7f03c-2e05-4c15-aa12-d1d114bb35df/tmcb_a_12277302_f0001.jpg)
TABLE 1 Effect of Hsp104 on the aggregation of HttpolyQ fragmentsTable Footnotea
FIG 2 Effects of different prions and concentrations of Htt fragments on the aggregation of HttpolyQ fragments. (A to D) Htt fragments on the GAL1 promoter, which were integrated into the chromosome, were induced overnight with galactose medium. (A and B) Maximized z-stack projections of HttQ103 (A) and HttQ103P (B) in [RNQ+] yeast. (C and D) maximized z-stack projections of HttQ103 (C) and HttQ103P (D) in [PSI+] yeast. (E and F) HttQ103 (E) and HttQ103P (F) fragments were expressed in [RNQ+] yeast from the CUP1 promoter grown in copper-free medium.
![FIG 2 Effects of different prions and concentrations of Htt fragments on the aggregation of HttpolyQ fragments. (A to D) Htt fragments on the GAL1 promoter, which were integrated into the chromosome, were induced overnight with galactose medium. (A and B) Maximized z-stack projections of HttQ103 (A) and HttQ103P (B) in [RNQ+] yeast. (C and D) maximized z-stack projections of HttQ103 (C) and HttQ103P (D) in [PSI+] yeast. (E and F) HttQ103 (E) and HttQ103P (F) fragments were expressed in [RNQ+] yeast from the CUP1 promoter grown in copper-free medium.](/cms/asset/485d64f4-32af-4e45-83e0-13335cf70696/tmcb_a_12277302_f0002.jpg)
FIG 3 Maximized z-stack images of HttQ103 fragments expressed in yeast cured of prions by guanidine as a function of time in galactose medium. (A to D) Yeast cells were grown in galactose medium for 1 to 4 days. (E and F) A colony from a galactose plate was resuspended in galactose medium and grown overnight in galactose medium (E), followed by growing cells overnight in 5 mM guanidine (F).
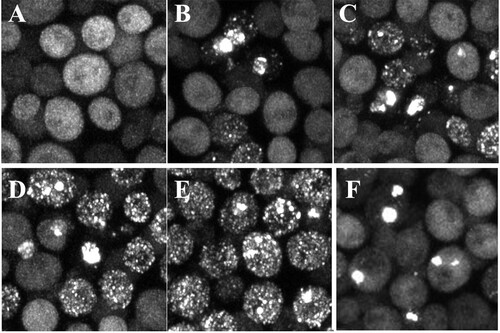
FIG 4 Maximized z-stack images of the effect of expressing different Hsp104(Y662) channel mutants on the aggregation of HttQ103. HttQ103 was expressed from the GAL1 promoter for 4 days in an hsp104 deletion strain transformed to express the different Hsp104(Y662) point mutants from the HSP104 promoter, as indicated.

FIG 5 Time course of the formation of HttQ103 aggregates in the absence and presence of Hsp104. (A) Time series obtained in an hsp104 deletion strain constitutively expressing HttQ103. (B) Time series obtained in an hsp104 deletion strain constitutively expressing HttQ103 following the induction of Hsp104 expression from the GAL1 promoter. (Top) No detectable aggregate was present in the cell at the start of the experiment; (bottom) a large aggregate was present in the cell at the start of the experiment. The arrow in the cell at the 30-min time point in the bottom panel indicates a flare-like projection coming off the aggregate. (See Movies S1 and S2 in the supplemental material for images acquired at 10-min intervals.) In these experiments, the yeast cells were immobilized on an agarose pad to acquire z-stack images of the same field at various times. Images are all maximized z-stacks.
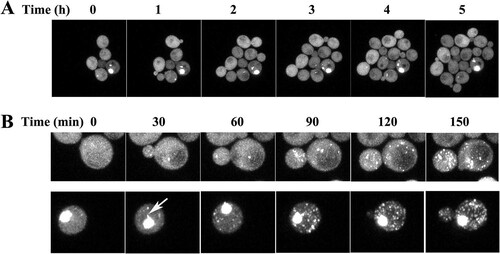
FIG 6 Dot blot and Western blot assays of HttQ103. (A) Dot blot of HttQ103 from yeast lysates that were either not treated with SDS buffer or boiled in 2% SDS. Lane 1, lysate prepared from [PRION+] yeast cells expressing HttQ103 from the GAL1 promoter, which was integrated into the chromosome; lane 2, lysate prepared from [prion−] yeast cells expressing HttQ103 from the GAL1 promoter integrated into the chromosome that were cultured on galactose plates and then grown on galactose medium; lane 3, lysate prepared from [PRION+] yeast cells expressing HttQ103 from the GAL1 promoter on a 2μm plasmid. The same concentration of total protein was added to each well. (B) Western blotting of HttQ103 from yeast lysates grown under different conditions. Lanes 1 and 3, total lysates that were boiled in SDS buffer; lanes 2 and 4, lysates treated with formic acid before boiling in SDS buffer. Lanes 1 and 2 are lysates prepared from [PRION+] yeast cells expressing HttQ103 from the GAL1 promoter integrated into the chromosome. Lanes 3 and 4 are lysates prepared from [prion−] yeast cells expressing HttQ103 from the GAL1 promoter integrated into the chromosome that were cultured on galactose plates and then grown on galactose medium. [PRION+] yeast contains both the [RNQ+] and [PSI+] prions. [prion−] yeast cells are cured of all prions.
![FIG 6 Dot blot and Western blot assays of HttQ103. (A) Dot blot of HttQ103 from yeast lysates that were either not treated with SDS buffer or boiled in 2% SDS. Lane 1, lysate prepared from [PRION+] yeast cells expressing HttQ103 from the GAL1 promoter, which was integrated into the chromosome; lane 2, lysate prepared from [prion−] yeast cells expressing HttQ103 from the GAL1 promoter integrated into the chromosome that were cultured on galactose plates and then grown on galactose medium; lane 3, lysate prepared from [PRION+] yeast cells expressing HttQ103 from the GAL1 promoter on a 2μm plasmid. The same concentration of total protein was added to each well. (B) Western blotting of HttQ103 from yeast lysates grown under different conditions. Lanes 1 and 3, total lysates that were boiled in SDS buffer; lanes 2 and 4, lysates treated with formic acid before boiling in SDS buffer. Lanes 1 and 2 are lysates prepared from [PRION+] yeast cells expressing HttQ103 from the GAL1 promoter integrated into the chromosome. Lanes 3 and 4 are lysates prepared from [prion−] yeast cells expressing HttQ103 from the GAL1 promoter integrated into the chromosome that were cultured on galactose plates and then grown on galactose medium. [PRION+] yeast contains both the [RNQ+] and [PSI+] prions. [prion−] yeast cells are cured of all prions.](/cms/asset/891d3c2d-4f4c-44cf-9467-06fd3bf2a148/tmcb_a_12277302_f0006.jpg)
