Figures & data
Figure 1. ET-1 promotes protein carbonylation in smooth muscle cells. Pulmonary artery smooth muscle cells were treated with ET-1 (30 nM) and harvested at the indicated time points. Cell lysates were prepared and derivatized with DNPH. Carbonylated protein levels were monitored by Western blotting with the DNP antibody. Coomassie blue staining showed no differences in total protein levels. The bar graph shows the mean ± SEM of the percentage of total carbonyl content relative to the untreated control as determined by densitometry. * denotes the value that is significantly different from the untreated control at P < 0.05 (n = 6), as determined by one-way ANOVA with a Student–Newman–Keuls post-hoc test. Reproduced from Wong et al. (2008)9 in accordance with the Copyright Transfer Agreement.
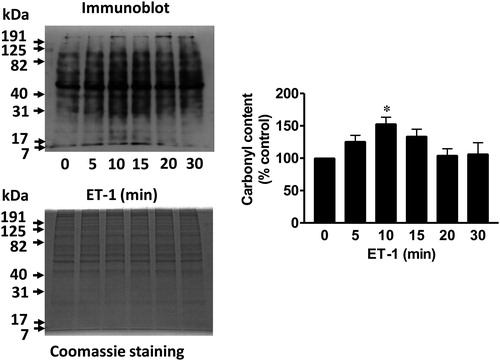
Figure 2. Evidence to support the hypothesis for the occurrence of primary protein carbonylation in ET-1 signaling. Pulmonary artery smooth muscle cells were treated with ET-1 (30 nM) and harvested at the indicated time points to prepare cell lysates as previously described [Wong et al., 2008]. (A) Cell lysates were subjected to Western blotting using 4-HNE and MDA antibodies. The bar graph shows the means ± SEM (n = 4) of the percentage of total 4-HNE or MDA adduct content relative to the untreated control as determined by densitometry. (B) Cell lysates (20 µg protein) were treated with 1% SDS, derivatized with biotin hydrazide (5 mM) for 2 hours by mixing at room temperature, incubated with sodium cyanoborohydride (15 mM) on ice for 30 minutes and precipitated with trichloroacetic acid (10%) on ice. Protein pellets were washed three times with ethanol:ethyl acetate before loading onto gels for Western blotting using avidin-horseradish peroxidase. * denotes significantly different values from untreated control at P < 0.05 (n = 4), as determined by one-way ANOVA with a Student–Newman–Keuls post-hoc test.
![Figure 2. Evidence to support the hypothesis for the occurrence of primary protein carbonylation in ET-1 signaling. Pulmonary artery smooth muscle cells were treated with ET-1 (30 nM) and harvested at the indicated time points to prepare cell lysates as previously described [Wong et al., 2008]. (A) Cell lysates were subjected to Western blotting using 4-HNE and MDA antibodies. The bar graph shows the means ± SEM (n = 4) of the percentage of total 4-HNE or MDA adduct content relative to the untreated control as determined by densitometry. (B) Cell lysates (20 µg protein) were treated with 1% SDS, derivatized with biotin hydrazide (5 mM) for 2 hours by mixing at room temperature, incubated with sodium cyanoborohydride (15 mM) on ice for 30 minutes and precipitated with trichloroacetic acid (10%) on ice. Protein pellets were washed three times with ethanol:ethyl acetate before loading onto gels for Western blotting using avidin-horseradish peroxidase. * denotes significantly different values from untreated control at P < 0.05 (n = 4), as determined by one-way ANOVA with a Student–Newman–Keuls post-hoc test.](/cms/asset/dc88c937-1d1a-4685-84f6-2bc5f58d7318/yrer_a_11653440_f0002_b.jpg)
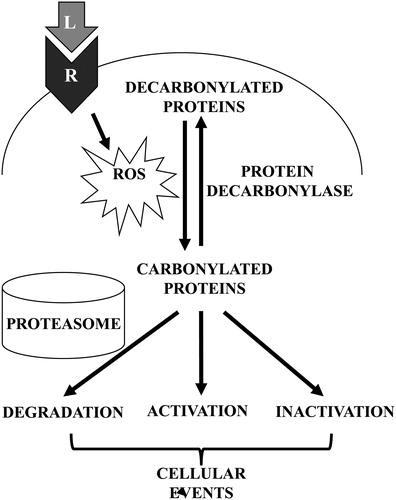
Figure 3. Schematics of proposed mechanisms involving primary protein carbonylation in cell signaling. Receptor (R) interactions with ligands (L) such as ET-1 trigger the generation of ROS, which in turn promote the metal-catalyzed formation of primary protein carbonyls. Carbonylated proteins may be selectively degraded or may influence the activities of signal transduction molecules to elicit cellular events. Primary protein carbonylation may be reversible by as-yet unidentified protein decarbonylases.