Figures & data
Figure 1. Protective role of paraoxonase 1 in cardiovascular disease and inflammation. Ischemic stroke is a very prevalent complication of atherosclerosis. PON1 may have a protective role in this disorder as it carries both antioxidant and anti-inflammatory functional properties. In this diagram we summarize the current knowledge on the main functional activities of PON1 a protective factor vis-a-vis atherogenesis. Reactive oxygen species issued from inflammation oxidize lipids in LDL (1) or cell membranes (2) producing oxidized phospholipids (3). PON1 produced in the liver and carried by HDL (4) exerts a lactonizing and lactonase activity (5) that results in the production of a carboxylic acid (5), eliminating further damage. The lactonase activity of PON1 also permits it to detoxify homocysteine thiolactone (6). This compound is associated with increased cardiovascular risk, since homocysteinylation of proteins (coagulation factors, lipoproteins, endothelial receptors) is atherogenic (7). Homocysteine thiolactone is one of the natural substrates of PON1, which hydrolyzes it to innocuous homocysteine (8). PON1 also exerts its salutatory action on macrophages and other inflammatory cells (9), preventing cellular oxidative stress and blocking cytokine cascades that aggravate inflammation that may lead to enhanced atherogenesis and neurovascular disease (10).
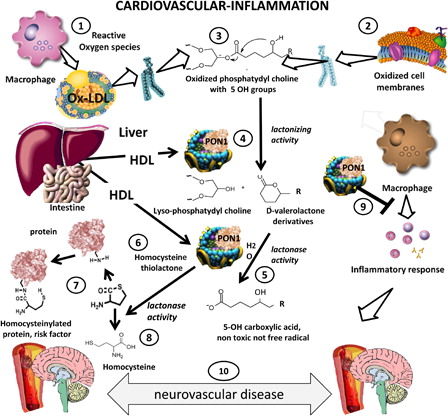
Figure 2. PON1 in toxicology: implications for neurodegenerative diseases. PON1 evolved from lactonases that have a key role in natural immunity, limiting quorum sensing lactones from bacteria. In evolution it acquired promiscuous esterase activities, hydrolyzing compounds of interest in toxicology. Organophosphate pesticides (1) are metabolized to their respective oxon by phase 1 metabolism in the liver (2,3). These oxons are toxic due to their inhibition of acetylcholinesterase, which is associated with several neurodegenerative disorders (4). PON1 hydrolyzes paraoxon (oxon from parathion, from which the name derives) and oxons from many other organophosphates (5) and detoxifies them. Major polymorphisms in PON1 significantly change this activity (6) and may be associated with increased susceptibility to neurodegenerative disorders as discussed in the text.
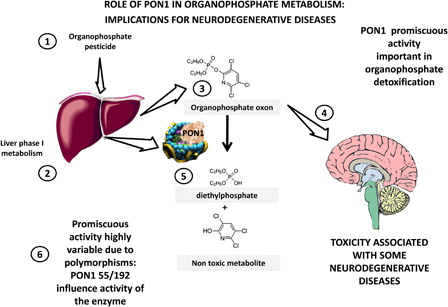
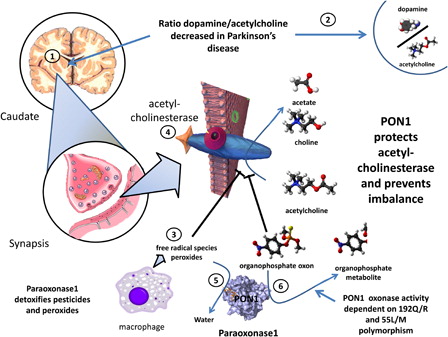
Figure 3. Putative involvement of PON1 in susceptibility to Parkinson's disease. In Parkinson's disease, due to death of degeneration in the substantial nigra there is an imbalance between acetylcholine and dopamine (1 and 2). Free radical species (3) that generate peroxide and organophosphate oxons both attack and inhibit acetylcholinesterase (4), thereby increasing acetylcholine and aggravating the imbalance. PON1, thorough its peroxidase (5) and triesterase (6) actions, blocks these effects and prevents the worsening of the imbalance. Of note, the oxonase activity of PON1 is highly dependent on the most common polymorphisms. Subjects with different phenotypes may thereby be more or less susceptible to damage to acetylcholine.