Figures & data
Figure 1. Innate immune response and host defense systems: The recognition of pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) located on the surface of host immune cells is a primitive part of the innate immune systems for protecting the host against invading pathogen. In the early phase of sepsis, an uncontrolled innate immune system enhances release of cytokines and generation of reactive oxygen species (ROS), which contribute to induce multiple organ failure and systemic inflammatory response syndrome (SIRS). Enhanced protective activity of the antioxidant network, cytokine neutralization or inhibition of lymphocyte growth might be of therapeutic potential in SIRS. In addition, optimal glucocorticoid secretion via HPA-axis stimulation in response to immune stress shows anti-inflammatory effects and enhances resistance against septic shock. Thus, the brain–endocrine–immune network might be important for the modulation of immunological balance during sepsis.
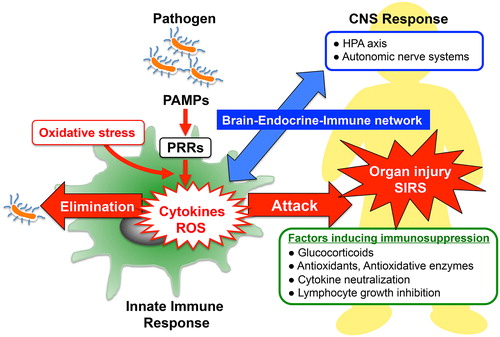
Figure 2. HPA-axis in response to LPS and cross-talk between the brain and immune system: The major part of the HPA-axis system is constituted by the hypothalamus, the pituitary gland, and the adrenal glands. When exposed to stressors, secretion of corticotropin-releasing factor (CRF) into hypophysial portal vessels stimulates the anterior pituitary gland thereby releasing adrenocorticotropic hormone (ACTH) into the circulation. Circulating ACTH binds to the type-2 melanocortin receptor (MC2R) in the adrenal cortex, where it stimulates glucocorticoid release. Elevated glucocorticoids in the circulation inhibit further activation of the HPA-axis through glucocorticoid receptor-mediated mechanisms in the brain so as to maintain homeostasis. Invading pathogens enhance glucocorticoid release that modulates the systemic immune response. TLR4-stimulated immune cells in response to LPS release excessive amounts of cytokines into the circulation, thereby stimulating the HPA-axis. In addition, LPS directly affects activation of the HPA-axis via the TLR4 signaling pathway exhibited in both the resident immune cells and neurons in the brain. Blue lines represent the HPA-axis to maintain homeostasis; blue dotted lines represent suppression of glucocorticoid release by negative feedback loops; red lines represent stimulation of the HPA-axis in response to LPS.
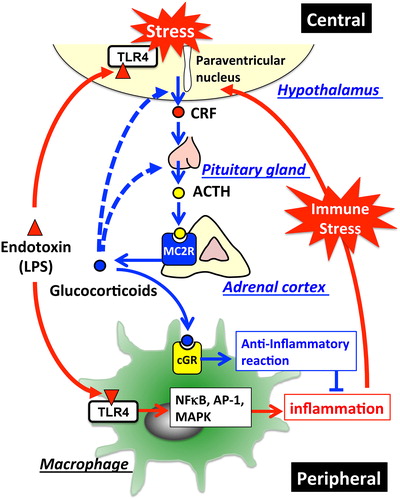
Figure 3. Variety of cellular responses to glucocorticoids and modulation of mitochondrial functions: Cellular responses to glucocorticoids are initiated by its binding to the cytosolic glucocorticoid receptors (GRs) in their inactive form. Homodimers of the GR complex bind to nuclear glucocorticoid response elements (nGREs) and activate the gene transcriptions driving the expression of anti-inflammatory genes including those encoding annexin A1, glucocorticoid-induced leucine zipper protein (GILZ), and mitogen-activated protein kinase phosphatase-1 (MKP-1) (transactivation). In addition, GR complex up-regulate the expression of uncoupling protein-2 (UCP2) thereby suppressing mitochondrial ROS generation. On the other hand, GRs may complex with transcription factors (TFs) such as NFκB and AP-1 thereby preventing them from binding to their target genes, including pro-inflammatory genes such as IL-1B, TNFA, and chemokines (transrepression). Mitochondrial generation of ROS enhances the activity of these TFs. Activated GRs also modulate mitochondrial functions in various cells including neurons, hepatocytes, and immune cells. The mitochondrial genomes of human and rat contain sequences similar to that of the nGREs. GREs in the mitochondrial genome (mGRE) modulate the biosynthesis of the proteins involved in oxidative phosphorylation (OXPHOS) by regulating mitochondrial gene expression. Furthermore, interactions of GRs with B-cell lymphoma-2 (Bcl-2), and mitochondrial thioredoxin (Trx2) regulate mitochondrial function including oxidation, energy production, and cellular survival.
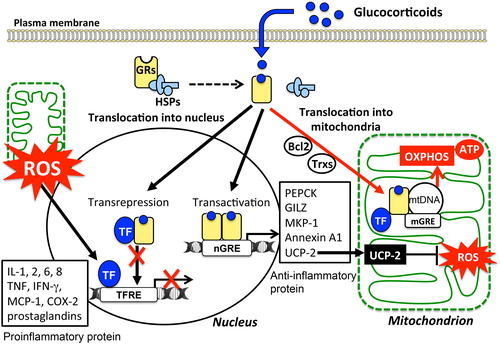
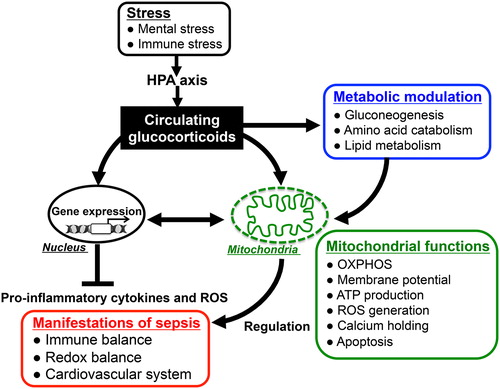
Figure 4. Glucocorticoid actions for immunomodulation via nucleus- and mitochondria-dependent signaling pathways: Glucocorticoids have pleiotropic effects on energy metabolism (gluconeogenesis, amino acid catabolism, fatty acid metabolism), mitochondrial activity, and immune responses. The alteration of mitochondrial functions such as oxidative activity, ATP production, and apoptotic signaling in response to glucocorticoids plays important roles in modulating host immune responses and redox systems. Thus, appropriately stimulated HPA-axis followed by glucocorticoid release in response to endotoxin-induced immune stress might reduce the parameters of inflammatory response and improve the mortality in sepsis through both nucleus- and mitochondria-dependent signaling pathways.