Figures & data
Figure 2 Continual absorbance decrease of ethanolic solution of DPPH radical (50 μmol/l) in the presence of 200 μM concentration of the compounds tested at λmax = 518 nm. (•) Compound 1 and (○) compound 2. The curves represent results from two typical experiments.
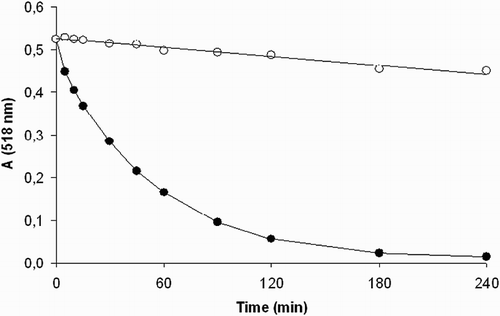
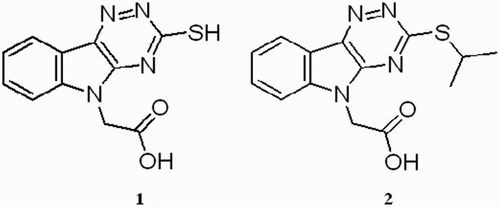
Table 1 DPPH test
Figure 3 Stoichiometry of DPPH scavenging by compound 1. Concentration dependence of DPPH absorbance decrease in the presence of increasing concentrations of 1. CDPPH = 50 μM, time of reaction 24 hours. Results are mean values ± SD from five experiments.
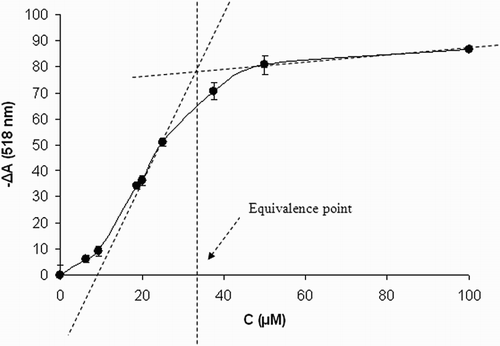
Figure 4 (A) Hemolysis curves induced by t-BuOOH. Erythrocyte suspensions (1.5%) were incubated with 250 μM t-BuOOH in the presence of 100 μM (-•-) or 1 mM (-▪-) 1. Control incubations (-Δ-). Each value is the mean ± SD from four to eight experiments. (B) Kinetics of t-BuOOH quenching by compound 1. t-BuOOH (250 µM) was incubated in the absence (-Δ-) or presence of 100 µM (-•-) or 1 mM (-▪-) compound 1 under the conditions of the hemolytic experiment omitting the erythrocytes. Results are mean values ± SD from three experiments.
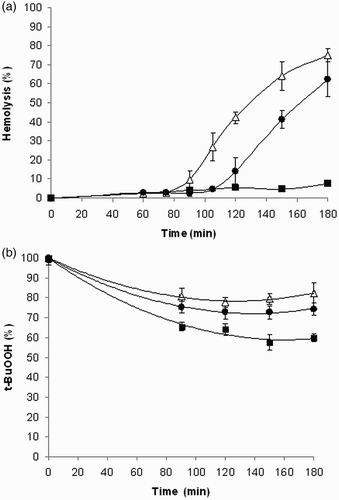
Figure 5 Time course of uptake of compound 1 by isolated rat erythrocytes. Compound 1 (A) 100 μM, (B) 1 mM was incubated with washed red blood cells (hematocrit of 20%) for indicated time intervals at 37°C. Experimental points represent mean values ± SEM from four independent experiments.
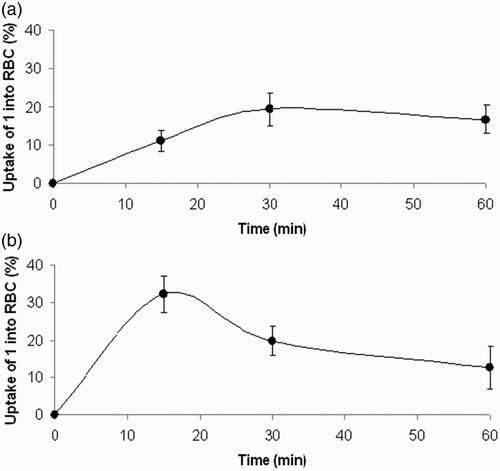
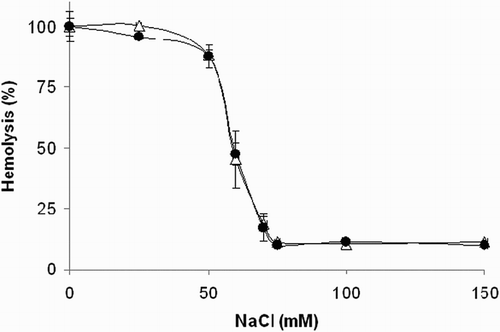
Figure 6 Effect of compound 1 on osmotic fragility of rat erythrocytes. Erythrocyte suspensions (0.4% hematocrit) in 10 mM phosphate buffer containing increasing concentrations of NaCl were incubated at 37°C for 1 hour in the absence (Δ) or presence (•) of compound 1 (250 μM). Experimental points represent mean values ± SD from at least three independent experiments.