Figures & data
Figure 1. Study flow of participant selection. HCV: hepatitis C virus; SVR: sustained virologic response.
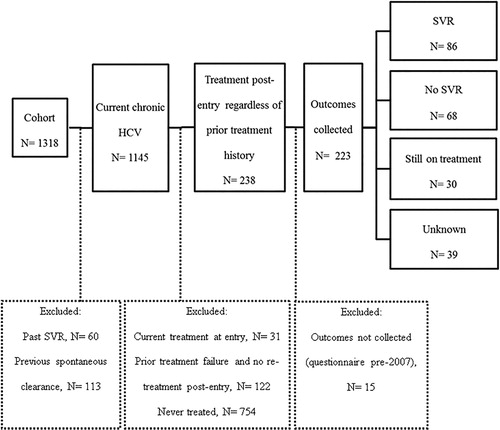
Table 1. Characteristics of Canadian Coinfection Cohort (CCC) participants overall (at cohort enrollment) and of participants beginning HCV treatment (at their pretreatment visit)
Table 2. Incidence rates of health services used (per 100 person-years) among SVR-achievers compared with non-responders
Figure 2. Proportion of patients reporting substance use over time (cohort entry, before treatment, 6 months after treatment, 1 year after treatment) comparing SVR-achievers and non-responders. SVR: sustained virologic response; pre-tx: pretreatment.
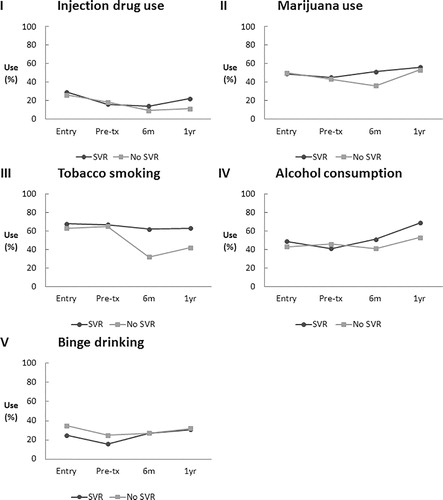
Figure 3. Liver fibrosis over time (cohort entry, before treatment, 6 months after treatment, 1 year after treatment) comparing SVR-achievers and non-responders. Aspartate aminotransferase to platelet ratio index (APRI) scores < 0.5 indicate no fibrosis. Aspartate aminotransferase to platelet ratio index scores ≥ 1.5 indicate significant fibrosis. Sustained virologic response was significantly associated with improved APRI scores 6 months and 1 year posttreatment after adjusting for pretreatment APRI scores: linear regression coefficient − 0.7 (95% CI: − 1.1, − 0.3) and ( − 0.6 [95% CI: − 1.1, − 0.1]), respectively. APRI: aspartate aminotransferase to platelet ratio index; SVR: sustained virologic response; Q1: first quartile; Q3: third quartile.
![Figure 3. Liver fibrosis over time (cohort entry, before treatment, 6 months after treatment, 1 year after treatment) comparing SVR-achievers and non-responders. Aspartate aminotransferase to platelet ratio index (APRI) scores < 0.5 indicate no fibrosis. Aspartate aminotransferase to platelet ratio index scores ≥ 1.5 indicate significant fibrosis. Sustained virologic response was significantly associated with improved APRI scores 6 months and 1 year posttreatment after adjusting for pretreatment APRI scores: linear regression coefficient − 0.7 (95% CI: − 1.1, − 0.3) and ( − 0.6 [95% CI: − 1.1, − 0.1]), respectively. APRI: aspartate aminotransferase to platelet ratio index; SVR: sustained virologic response; Q1: first quartile; Q3: third quartile.](/cms/asset/6c632d08-6d0c-4083-a09f-575aaa5c875a/yhct_a_11732837_f0003_b.jpg)
