Figures & data
Figure 1. Mean (arithmetic mean) plasma concentration–time curves of oxycodone at steady state after oral administration of oxycodone 10 mg once daily (test, ◊) or oxycodone 5 mg twice daily (reference, ♦) under fasting conditions in 36 healthy subjects (for details see ScheidelCitation13).
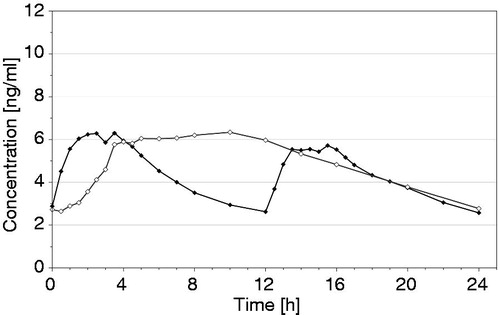
Figure 2. Study design for testing non-inferiority of oxycodone once daily (test) versus oxycodone twice daily (reference) at identical total daily doses.
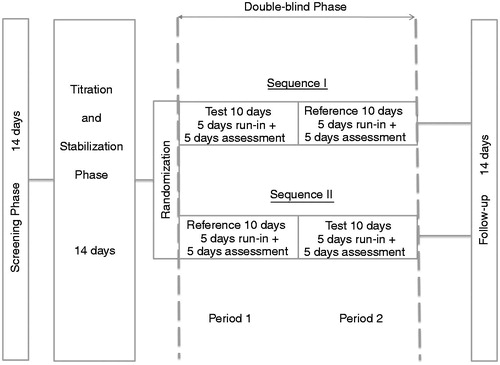
Table 1. Patient demographics at baseline for sequence I (test–reference), sequence II (reference–test) and total (safety) data set.
Table 2. Primary efficacy outcome, mean current pain (mm on 0 to 100 mm VAS) over the last 5 days of each treatment period for total as well as for period 1 and 2 of the cross-over, double-blind treatment phase of the study in the per protocol data set (n = 46).
Table 3. Statistical evaluation of primary efficacy parameter, difference in mean current pain (mm VAS on 0 to 100 mm VAS) over the last 5 days of each treatment period between oxycodone once daily (T) and oxycodone twice daily (R) at equal total daily doses, for complete population as well as subpopulations in different study parts (at/after interim analysis, part 1/2) and for different types of pain (malignant, non-malignant) in the per protocol data set (n = 46).
Figure 3. Daily mean current pain (mm on 0 to 100 mm VAS, mean with 95% CI) over the last five treatment days for oxycodone once daily (□) and oxycodone twice daily (•) at identical total daily doses in the full analysis data set (n = 60).
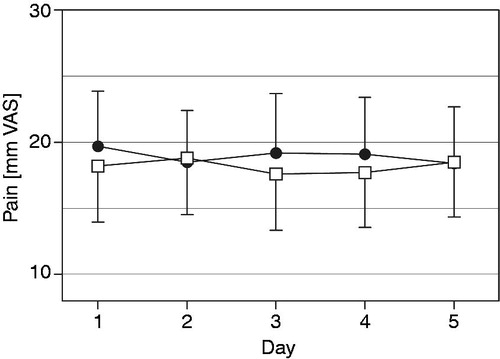
Figure 4. Current pain (mm on 0 to 100 mm VAS, mean with 95% CI) over the last five treatment days for oxycodone once daily (OOD) and oxycodone twice daily (OTD) at identical total daily doses for two of the five daily time points of pain evaluation (□ = evaluated at 08:00 h,♦ = evaluated at 20:00 h) in the full analysis data set (n = 60).
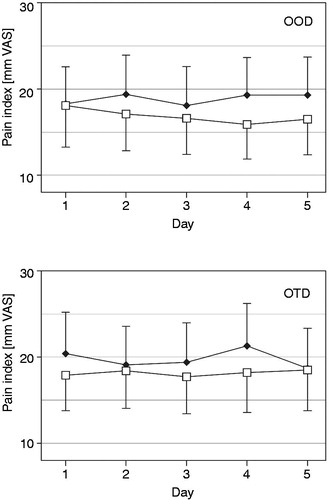
Table 4. Number of subjects (n and %) as well as incidence (nAE) of adverse events by system organ class (≥4.4%) and preferred term (>1.5%) in the safety data set (n = 68).
Figure 5. Nausea (mm on 0 to 100 mm VAS, mean with 95% CI) in the morning (A) and evening (B) over the last five treatment days for oxycodone once daily (□) and oxycodone twice daily (•) at identical total daily doses in the safety data set (n = 68).
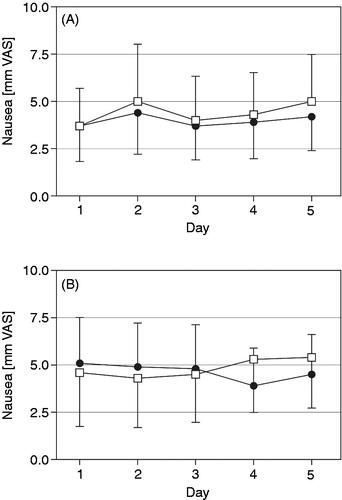
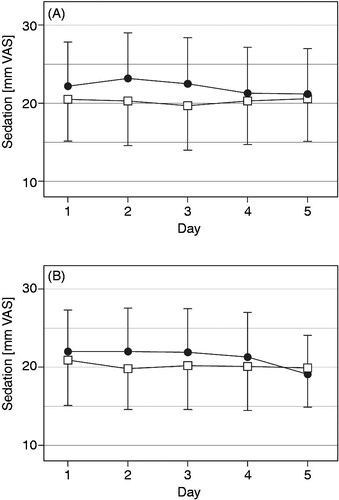
Figure 6. Sedation (mm on 0 to 100 mm VAS, mean with 95% CI) in the morning (A) and evening (B) over the last five treatment days for oxycodone once daily (□) and oxycodone twice daily (•) at identical total daily doses in the safety data set (n = 68).