Figures & data
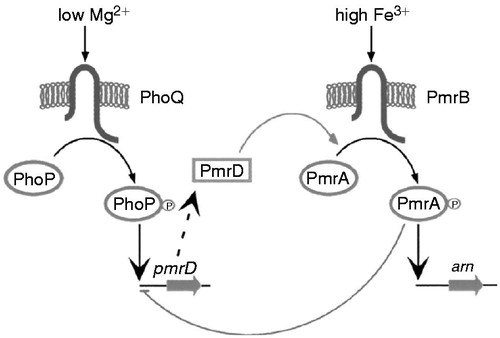
Figure 1. Colistin structures. (A) Structures of colistin A and B. (B) Structures of colistimethate A and B. Fatty acid: 6-methyloctanoic acid for colistin A and 6-methylheptanoic acid for colistin B; Thr = threonine; Leu = leucine; Dab = α,γ-diaminobutyric acid; α and γ indicate the respective amino groups involved in the peptide linkage. (Modified from Li et al.Citation4).
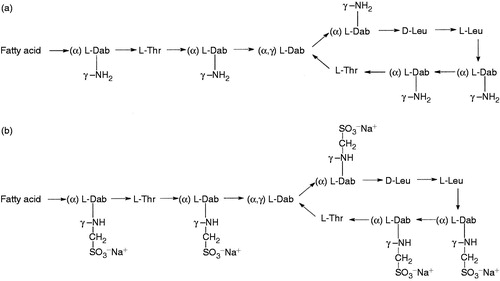
Figure 2. Pharmacokinetic (PK) simulation of colistin A and B in the in vitro PK model study (mean ± SD, n = 3). (From Zhao et al.Citation151).
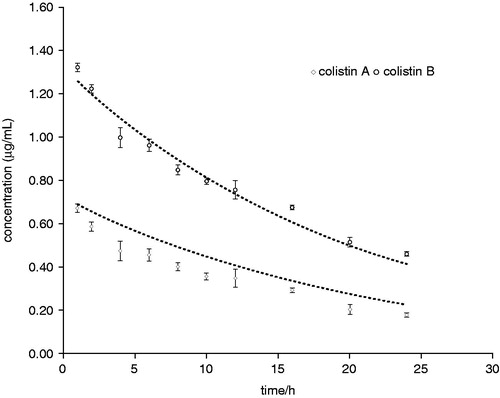
Figure 3. Action of colistin on bacterial membrane. The cationic cyclic decapeptide structure of colistin binds with the anionic LPS molecules by displacing calcium and magnesium from the outer cell membrane of Gram-negative bacteria, leading to permeability changes in the cell envelope and leakage of cell contents. By binding to the lipid A portion of LPS, colistin also has an anti-endotoxin activity. Disruption of the membranes should promote permeability for more conventional anti-pseudomonals. LPS: lipopolysaccharides; PBP: penicillin-binding protein. (From Martis et al.Citation58).
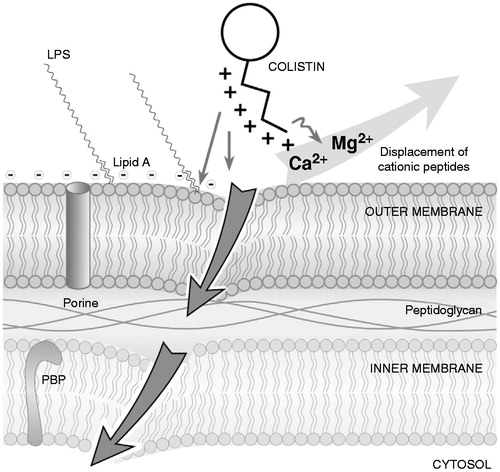
Figure 4. PmrA/PmrB and PhoP/PhoQ two-component regulatory systems. (From Falagas et al.Citation53).