Figures & data
Figure 1. Manufacturing changes for biotechnology products were categorized as low/moderate/high riskCitation1. Adapted from Lee et al. Comparability and biosimilarity: considerations for the healthcare provider. CMRO 2012;28:1053-8.

Figure 2. Number of manufacturing changes for monoclonal antibodies in their European Public Assessment Reports according to risk category (during the search period all non-proprietary names relate only to the trade named medicines listed in ).
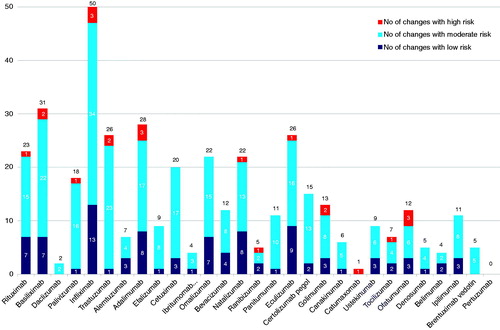
Figure 3. Average number of manufacturing changes per product year elapsed since registration (during the search period all non-proprietary names relate only to the trade named medicines listed in ).
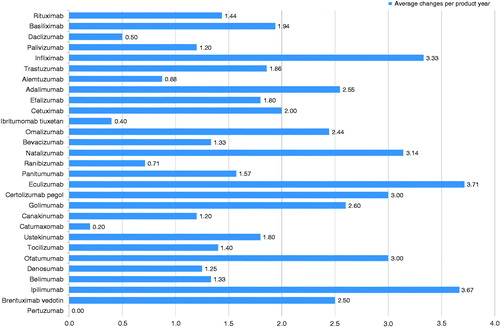
Table 1. Monoclonal antibody medicines and their EPARTable Footnote* ‘Procedural steps…’ documents from EMATable Footnote** webpage during the search periodCitation10.
