Figures & data
Table 1 Characteristics of participants with high (HCF) or medium cardiorespiratory fitness (MCF); and with high (HNF) or medium neuromuscular fitness (MNF)
Fig. 1 Means and SD of time to exhaustion with caffeine (solid bars) and placebo (hatched bars). Participants were divided according to cardiorespiratory (left) or neuromuscular fitness (right), with high groups shown in black and medium groups shown in grey. There was a significant treatment effect (p < 0.001)
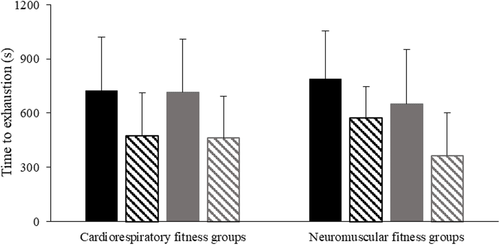
Fig. 2 Means and SD of countermovement jump height with caffeine (solid bars) and placebo (hatched bars). Participants were divided according to cardiorespiratory (a) or neuromuscular fitness (b), with high groups shown in black and medium groups shown in grey. One to 5 correspond to time points as follows: 1, before the start of the trial; 2, between the 1st and 2nd periods; 3, immediately after the end of the 2nd period; 4, immediately before the start of the 3rd period; 5: between the 3rd and 4th periods. There were significant treatment and time effects in both a and b, as well as a significant group effect (by design) in b (p < 0.05)
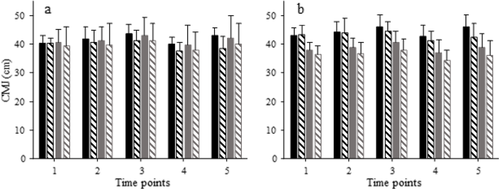
Table 2 Mean arterial pressure for the cardiorespiratory and neuromuscular fitness classifications (mm Hg, mean ± SD)
Table 3 Heart (HR) and rating of perceived exertion (RPE) for the cardiorespiratory and neuromuscular fitness classifications (mean ± SD)
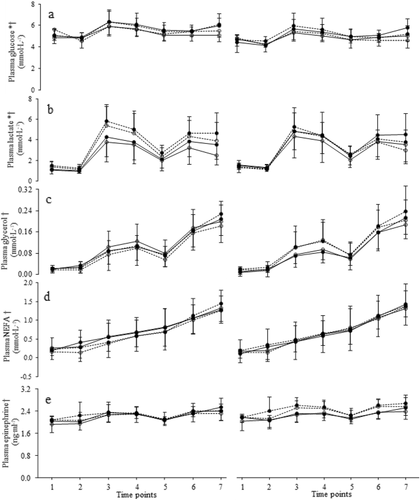
Fig. 3 Means and SD of plasma glucose (a), lactate (b), glycerol (c), NEFA (d) and epinephrine (e) with caffeine (full circles) and placebo (open circles). Participants were divided according to cardiorespiratory (left) or neuromuscular fitness (right), with high groups shown with solid lines and medium groups shown with dashed lines. One to 7 correspond to time points as follows: 1, 75 min before the start of the trial; 2, 15 min before the start of the trial; 3, between the 1st and 2nd periods; 4, immediately after the end of the 2nd period; 5, immediately before the start of the 3rd period; 6: between the 3rd and 4th periods; 7, at exhaustion. *Significant treatment effect (p ≤ 0.01). †Significant time effect (p < 0.001)
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
