Figures & data
Table 1 Select results on oxytocin quality in LMICs
Fig. 1 Map of WHO Member States and the Required Temperature and Relative Humidity Conditions for Stability Testing of Non-Refrigerated Pharmaceuticals [Citation14] In 2015, WHO developed an updated list of WHO member states and the temperature and relative humidity conditions at which stability testing of pharmaceutical products must be conducted to allow non-refrigerated storage in that country, as illustrated in the map (above)
![Fig. 1 Map of WHO Member States and the Required Temperature and Relative Humidity Conditions for Stability Testing of Non-Refrigerated Pharmaceuticals [Citation14] In 2015, WHO developed an updated list of WHO member states and the temperature and relative humidity conditions at which stability testing of pharmaceutical products must be conducted to allow non-refrigerated storage in that country, as illustrated in the map (above)](/cms/asset/48f13174-c0a5-4329-98e6-3e40d6af72af/jppp_a_12315138_f0001.png)
Fig. 2 Stability study comparing content of oxytocin 10 IU/mL injection ampoules labelled for storage at < 25 °C with those labelled for storage at 2–8 °C when stored at 30 °C for 120 days [Citation24]
![Fig. 2 Stability study comparing content of oxytocin 10 IU/mL injection ampoules labelled for storage at < 25 °C with those labelled for storage at 2–8 °C when stored at 30 °C for 120 days [Citation24]](/cms/asset/4ddc9f58-228b-432c-bd60-d0e7b1f00a4b/jppp_a_12315138_f0002.png)
Table 2 Select pharmacopeia monograph quality specifications for oxytocin API and FPP
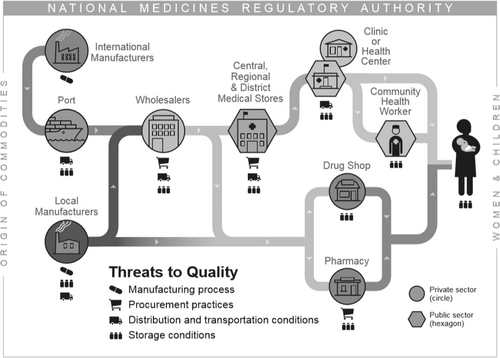
Fig. 3 Threats to Oxytocin Quality Occur throughout the Supply Chain for Oxytocin. Threats to oxytocin quality occur throughout manufacturing, procurement, distribution, and storage
Availability of data and materials
Not applicable.
