Figures & data
Table 1 Categorisation of medicines and vaccines in the Ugandan Essential Medicine List (MoH 2012)
Table 2 Number and percentage of essential medicines not registered with the NDA according to VEN classification and the level of use in the health system
Fig. 1 Countries of origin for medicines on the Ugandan National Drug Register, 2012. PIC/S countries: Germany, UK, Cyprus, South Africa, Belgium, France, Malaysia, Switzerland, Indonesia, Italy, Sweden, Canada, The Netherlands, Greece, Spain, USA, Portugal, Denmark, Hungary, Slovenia, Korea, Finland, and Japan. Non-PIC/S countries (importing < 4% of products into Uganda): Pakistan, Egypt, Jordan, Morocco, Turkey, Bangladesh, Iran, and UAE
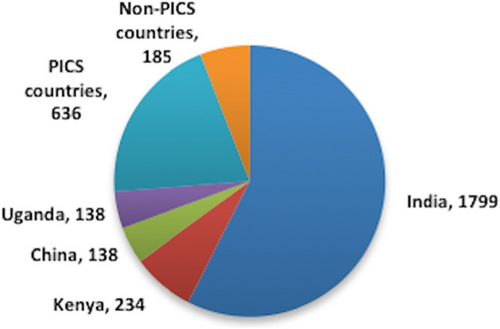
Table 3 Local production of essential medicines in Uganda
Additional file 1. Resolution of differences between the Ugandan EML and NDR.
Download MS Word (15.5 KB)Additional file 2. Key informants.
Download MS Word (14.3 KB)Additional file 3. Interview guide regulators.
Download MS Word (20.4 KB)Additional file 4. Interview guide procurers/distributors.
Download MS Word (17.8 KB)Additional file 5. Interview guide donors/NGOs.
Download MS Word (16.7 KB)Additional file 6. Interview guide regulators follow up.
Download MS Word (16.2 KB)Availability of data and materials
All data used in quantitative analysis were sourced from publicly available sources and are available from the corresponding author on reasonable request.
Full transcripts arising from this study are not publicly available due to concerns about identifying participants. Participants of this study did not provide consent for transcripts to be publicly shared or used in another project. We are following our ethics procedures for the AMASA project, for which institutional ethical approval was given. For the purposes of validating and verifying our data requests by qualified researchers may be directed to the corresponding author.
