Figures & data
Fig. 2 WHO five-step capacity-building model for the National Regulatory Authorities. Source: [Citation11]
![Fig. 2 WHO five-step capacity-building model for the National Regulatory Authorities. Source: [Citation11]](/cms/asset/64892c38-b590-452e-8e59-243984549baf/jppp_a_12315188_f0002.png)
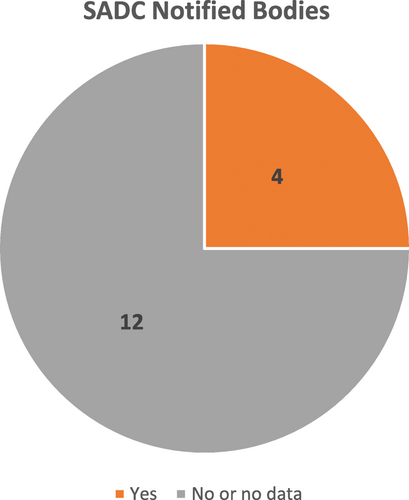
Fig. 3 Number of countries according to the type of NMRA Governance Structure, countries within SADC (N = 12), 2017
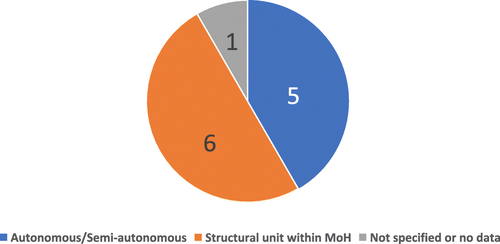
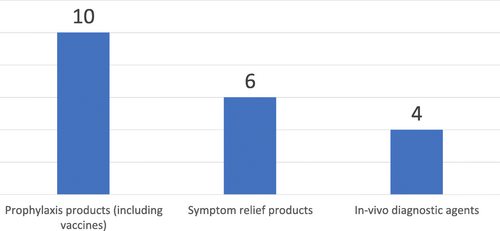
Fig. 4 Number of countries according to the scope of Medical Product Regulation, by type of Medical Product, Countries within SADC (N = 12), 2017
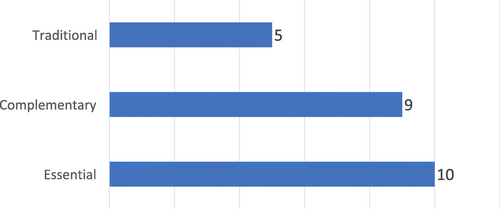
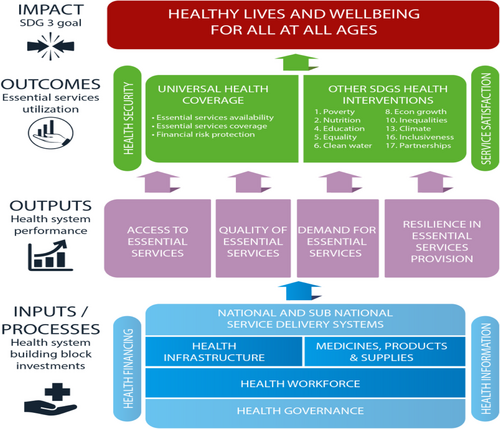
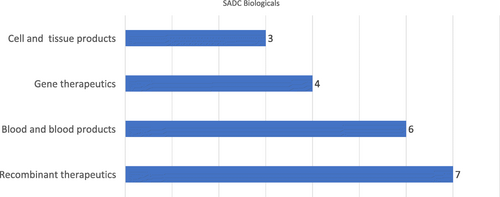
Fig. 5 Number of countries according to the scope of biologicals regulation, by type of biological product, countries within SADC (N = 12), 2017
Availability of data and materials
The datasets used and analysed during the study that are the main basis for the review are available from the corresponding author on request.