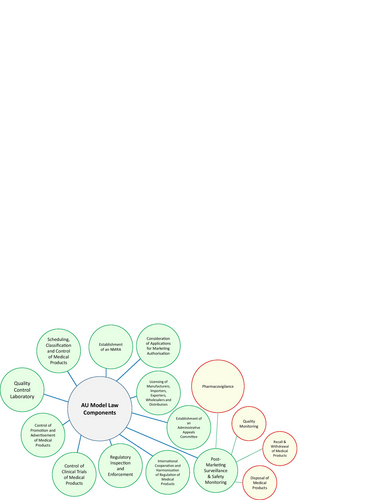
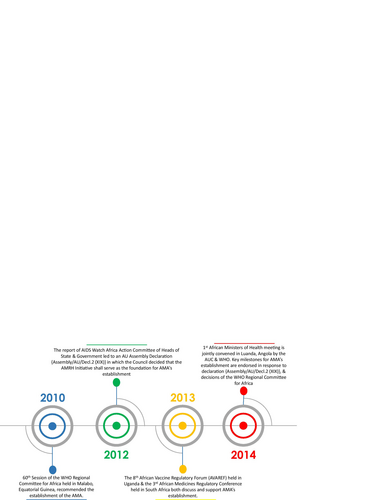
Figures & data
Fig. 3 The proposed structure of the AMA. The proposed governance structure of AMA includes Member States, a Board with strong African government and technical representation, key stakeholders and a Secretariat led by the Head of AMA. AMA’s Board will be responsible for strategic oversight and direction, financial performance and accounts to the Member States through the AUC. The Secretariat will be responsible for operational performance, strategy/business plan implementation, as well as coordination/facilitation of medicines regulatory activities and harmonisation. The AMA’s structure intends to ensure the maintenance of a lean staff and the use of both internal staff and experts from participating NMRAs. The role of key staff will therefore be the coordination of AMA’s activities. The European Medicines Agency (EMA) and WHO PQTm have used similar approaches. The Head of AMA will be supported by a resource mobilisation team, an advocacy and partnership team, a legal services team and a technical capacity team [Citation11]
![Fig. 3 The proposed structure of the AMA. The proposed governance structure of AMA includes Member States, a Board with strong African government and technical representation, key stakeholders and a Secretariat led by the Head of AMA. AMA’s Board will be responsible for strategic oversight and direction, financial performance and accounts to the Member States through the AUC. The Secretariat will be responsible for operational performance, strategy/business plan implementation, as well as coordination/facilitation of medicines regulatory activities and harmonisation. The AMA’s structure intends to ensure the maintenance of a lean staff and the use of both internal staff and experts from participating NMRAs. The role of key staff will therefore be the coordination of AMA’s activities. The European Medicines Agency (EMA) and WHO PQTm have used similar approaches. The Head of AMA will be supported by a resource mobilisation team, an advocacy and partnership team, a legal services team and a technical capacity team [Citation11]](/cms/asset/26b0d279-7102-475d-81fc-8d1474a1f518/jppp_a_12315229_f0003.png)
Table 1 Level of implementation of regulatory functions at national, regional and continental level [Citation11]