Figures & data
Table 1 Count of post-approval new indications per drug
Fig. 1 Probability of new indication exclusivity granted by FDA for drugs approved, 1997–2020. The probability of a new indication addition is highest when NMEs have been relatively recently (within 1–2 years) and when generic entry is around 7–8 years into the future. The probability is lowest when an NME has been approved for more than 15 years and when generic entry has already occurred. “Age (in years)” = number of years since FDA approval
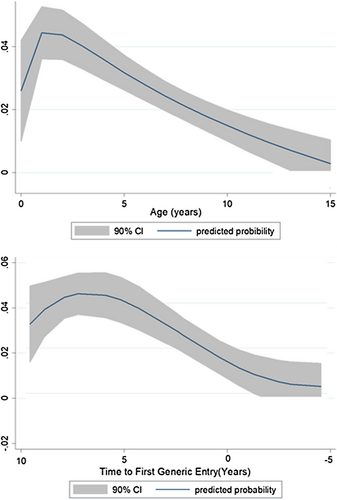
Fig. 2 Estimated regression coefficients. The imminence of generic entry negatively impacts the chances of a new indication being developed for an NME. Holding the effect of age constant, generic entry’s influence upon reducing the chances of a new indication addition is strongest when generic has already occurred and weakest when generic entry is still 5–10 years into the future
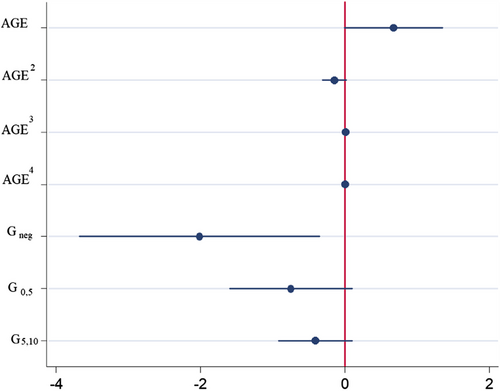
Fig. 3 Predicted conditional probability of a new indication exclusivity approval. When we applied the strongest observed influence of generic entry (i.e., generic entry had already occurred []) to all NMEs, shown in black is our model’s predicted probability of new indications additions, which is near zero, regardless of the age of the drug. Note that by law, generic entry cannot occur in the United States prior to 5 years after FDA approval when the product in question is designated as a small-molecule NME, and therefore, the black line begins only after 5 years accordingly. When we applied the weakest observed influence of generic entry (i.e., generic entry is 5–10 years into the future [
]) to all NMEs, shown in red is our model’s predicted probability of new indications additions, which is substantially higher, regardless of the age of the drug
![Fig. 3 Predicted conditional probability of a new indication exclusivity approval. When we applied the strongest observed influence of generic entry (i.e., generic entry had already occurred [β1]) to all NMEs, shown in black is our model’s predicted probability of new indications additions, which is near zero, regardless of the age of the drug. Note that by law, generic entry cannot occur in the United States prior to 5 years after FDA approval when the product in question is designated as a small-molecule NME, and therefore, the black line begins only after 5 years accordingly. When we applied the weakest observed influence of generic entry (i.e., generic entry is 5–10 years into the future [β3]) to all NMEs, shown in red is our model’s predicted probability of new indications additions, which is substantially higher, regardless of the age of the drug](/cms/asset/f334389c-44af-4729-bc07-e56ab0edac55/jppp_a_12315203_f0003.png)
Table 2 Real and counterfactual number of new indications additions
Table 3 Frequency of secondary indications by disease area
Additional file 1: Figure A-1.
Download PNG Image (47.4 KB)Additional file 2: Appendices.
Download MS Word (34.7 KB)Availability of data and materials
This study is not reporting data from a trial. All raw data are publicly available from the United States Food and Drug Administration.
