Figures & data
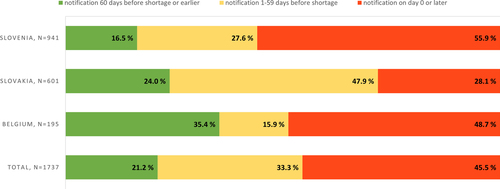
Fig. 1 Temporary drug shortage notification times in days by country during January 2020–November 2022, with the exception of Belgium where the observation period was July 2022–November 2022. Permanent market withdrawals not included
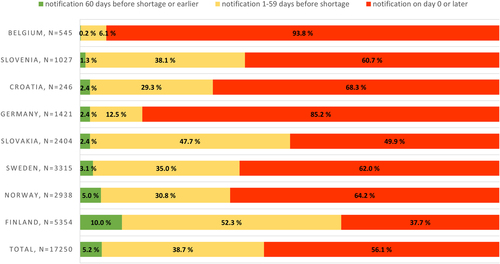
Fig. 2 Temporary drug shortage notification times for production-based vs. other temporary shortages (production-related shortage notification data separated in data for Croatia, Germany, and Norway, observation period January 2020–November 2022, for Belgium July 2022–November 2022). PROD = production-related temporary drug shortages; OTHER = temporary drug shortages caused by other than production-related reasons
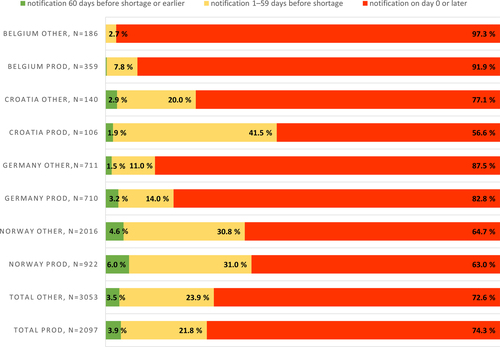
Fig. 3 Notification times of temporary drug shortages before, during and after the period of using the progressive notification fees in Finland, observation period in total from January 2020 to November 2022
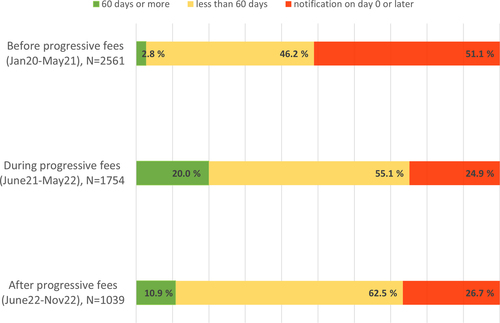
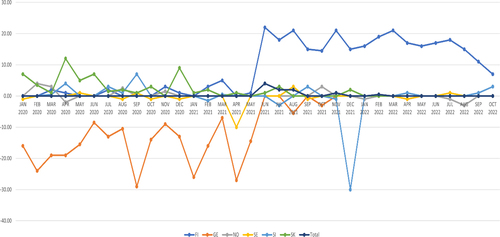
Fig. 4 Monthly median notification times in days for temporary drug shortages in Finland, Germany, Norway, Sweden, Slovakia, Slovenia, observation period January 2020–October 2022. In Finland, the progressive notification fees were in effect between June 2021 and May 2022. Permanent market withdrawals not included. (FI-Finland, GE = Germany, NO-Norway, SE = Sweden, SI = Slovenia, SK = Slovenia)
Availability of data and materials
The original data are public and obtainable from national medicines authorities: Belgian Federal Agency for Medicines and Health Products (FAMHP), Agency for medicinal products and medical devices of Croatia (HALMED), Finnish Medicines Agency (Fimea), German Federal Institute for Drugs and Medical devices (BfArM), Norwegian Medicines Agency, and Slovakian State Institute for Drug Control (SUKL), The Agency for Medicinal Products and Medical Devices of the Republic of Slovenia (JAZMP) and Swedish Medical Products Agency (Läkemedelsverket). The datasets analysed during the current study are also available upon reasonable request to the corresponding author.