Figures & data
Fig. 1 mRNA COVID-19 vaccines mechanism of action. Simplified graphical representation of the mode of action of mRNA vaccines against COVID-19. AAAAAA; poly-A tail of adenine nucleotides, APC; antigen presenting cells, CD; cluster of differentiation, LNP; lipid nanoparticles, mRNA; messenger ribonucleic acid, modRNA; modified ribonucleic acid, S; spike, SARS-CoV-19; severe acute respiratory syndrome coronovirus 2019, UTR; untranslated region. Image reproduced from Thomas SJ et al., Vaccine 2022;40(10):1483–92 (https://doi.org/10.1016/j.vaccine.2021.12.046) under Creative Commons CC-BY-NC-ND public licence available at https://creativecommons.org/licenses/by-nc-nd/4.0/
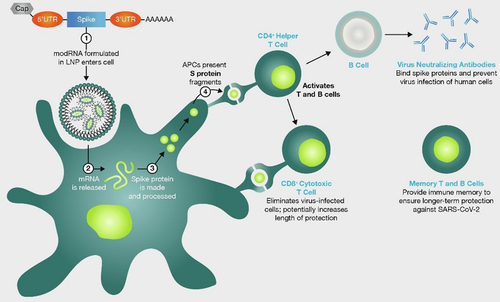
Fig. 2 mRNA COVID-19 vaccines milestones. Timeline showing selected epidemiological, clinical and regulatory events during the first year of the COVID-19 pandemic with an emphasis on Switzerland. WHO; World Health Organization
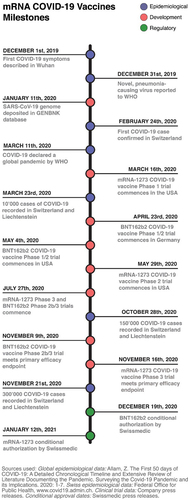
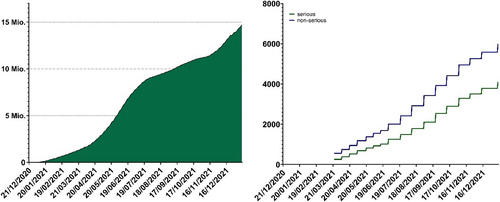
Fig. 3 Vaccine administration and adverse vaccination reaction reporting in Switzerland and Liechtenstein. Left: cumulative total administered doses of mRNA vaccines from the start of the Swiss vaccination program to the end of 2021 (mRNA-1273 and BNT162b2 were the only conditionally approved mRNA COVID-19 vaccines during this perioxd). Federal Office of Public Health, www.covid19.admin.ch. Right: cumulative totals of adverse vaccination reactions (blue; non-serious, green; serious) following mRNA vaccine administration reported to Swissmedic during the same period. Data on adverse vaccination reactions were generally released weekly during this period.
Source: Federal Office of Public Health https://www.covid19.admin.ch/api/data/20220705-0r3tf4ch/sources/COVID19VaccSymptoms.csv. Accessed 15 Nov 2023
Availability of data and materials
Data supporting this review was taken from public repositories and sources are given in the legends of the respective figures.
