Figures & data
Figure 1. Insulin sensitizer families. The family pedigrees of both TZDs (left panel) and non-TZDs are shown. Ciglitazone was the first insulin sensitizer tested in clinical trials. Troglitazone, rosiglitazone, and pioglitazone are the only insulin sensitizers ever to be approved for treatment of type 2 diabetes. Representative non-TZDs are shown on the right side of the page. Most of these compounds were modeled after Takeda's AD-5075, which was the most potent of the original Takeda analogs at differentiating 3T3L1 preadipocytes Citation[9], each with various substitutions for the TZD ring. None of these compounds were ever approved. Aleglitazar was the most recently discontinued.
![Figure 1. Insulin sensitizer families. The family pedigrees of both TZDs (left panel) and non-TZDs are shown. Ciglitazone was the first insulin sensitizer tested in clinical trials. Troglitazone, rosiglitazone, and pioglitazone are the only insulin sensitizers ever to be approved for treatment of type 2 diabetes. Representative non-TZDs are shown on the right side of the page. Most of these compounds were modeled after Takeda's AD-5075, which was the most potent of the original Takeda analogs at differentiating 3T3L1 preadipocytes Citation[9], each with various substitutions for the TZD ring. None of these compounds were ever approved. Aleglitazar was the most recently discontinued.](/cms/asset/6e3f478f-8380-4f91-9631-563cf1e8ccc3/ieid_a_839659_f0001_b.jpg)
Figure 2. Framework to view insulin-sensitizing pharmacology by modification of mTOT. The binding site for TZDs in the mitochondria is a complex in the inner mitochondrial membrane that has been called mTOT (mitochondrial target of thiazolidinediones). Key components of the complex are Mpc2, which is crosslinked by the drug analog crosslinker upon binding to intact membranes, and Mpc1, both of which are components of the pyruvate carrier. Regulation of the complex may determine the rate and delivery of pyruvate to help define the course of oxidative metabolism. The downstream effects that are known to occur upon the addition of the insulin sensitizers to various cell types include activation of AMPK, reduction of the activity of mTOR, and a metabolic break on Wnt signaling. These pathways are known to improve insulin signaling, but the consequences may vary in different cell types.
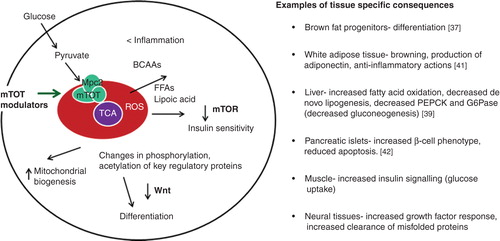
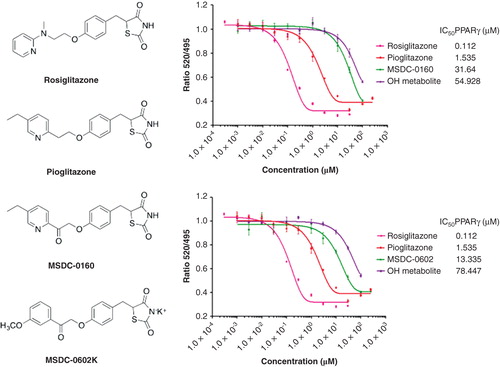
Figure 3. Relative effects of mTOT-modulating insulin sensitizers in development on binding to PPARγ. The structures of the two mTOT-modulating insulin sensitizers currently in clinical development are shown relative to rosiglitazone and pioglitazone. On the right side of the figure, the competition of the compounds for binding to human PPARγ is shown. For both MSDC-0160 (upper graph, right) and MSDC-0602 (lower graph, right), this activity is also shown for its major circulating reduced (OH) metabolite.