Figures & data
Figure 1. Daratumumab directly inhibits survival of CD138+ patient multiple myeloma cells. MM1S cells were treated with DARA with goat anti-human immunoglobulin for 2 days, followed by CellTiter-Glo luminescent cell viability assay (A) or Caspase 3/7 assay (B). (C) Freshly isolated CD138+ patient myeloma cells were treated with DARA (1, 10 g/ml) or isotype control (iso, 10 g/ml) in the presence of cross-linking Abs for 2 days. Percentages of Annexin V+/PI+ were determined by flow cytometry. Annexin V+ and combined Annexin V+/PI+ cells increased from 7.7 to 20.8% and 10.9 to 15.4%, indicating that DARA directly induces apoptosis of patient MM cells. (D) Light microscopy showed homotypic adhesion and aggregation of MM patient cells following DARA treatment.
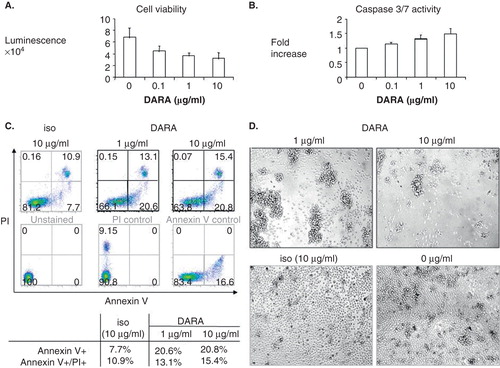
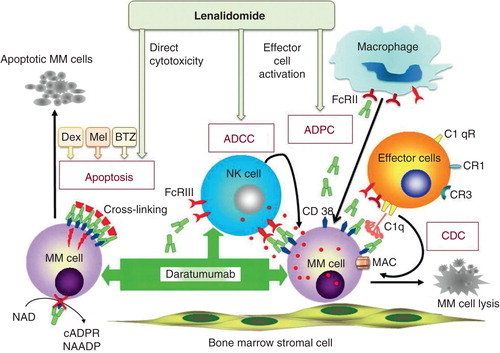
Figure 2. Mechanisms of action of daratumumab against multiple myeloma cells in the bone marrow microenvironment. DARA induces anti-MM effects via multiple mechanisms. It directly induces MM cell apoptosis when cross-linked with anti-human immunoglobulin or FcγRs expressed on various effector cells. DARA-induced inhibition of CD38 ADPR-ribosyl cyclase activity may also contribute to its direct killing of MM cells. Furthermore, antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADPC) and complement-mediated cytotoxicity (CDC) also contribute to DARA-induced MM cell death. ADCC and ADPC is achieved via binding of FcγRs on NK cells and macrophages (myeloid effector cells) by tumor cell-bound DARA. CDC is dependent on the interaction of the antibody Fc with the classic complement-activating protein C1q, resulting in the accumulation of C3b. Antibody opsonization and activation of complement leads to phagocytosis of tumor cells. C3b also binds to C3 convertase to form C5 convertase, inducing the membrane attack complex (MAC) that forms transmembrane channels. These channels in turn disrupt the phospholipid bilayer of MM cells, leading to cell lysis and death. DARA-induced anti-MM activities can be further enhanced by lenalidomide via both direct and indirect killing of MM cells. Dexamethasone (Dex)/prednisolone, bortezomib (BTZ) or melphalan (mel) also augment direct toxicity induced by DARA.