Figures & data
Table 1. Available radiotracers and corresponding half-lives, decay mode and major γ emission energy for NIS-mediated imaging with SPECT of PET instrumentation.
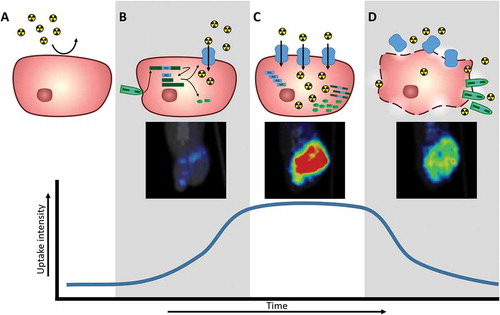
Table 2. Table of oncolytic viruses expressing the NIS transgene to mediate radiotracer uptake for in vivo nuclear imaging monitoring of infection, separated by oncolytic virus and tumor type.**
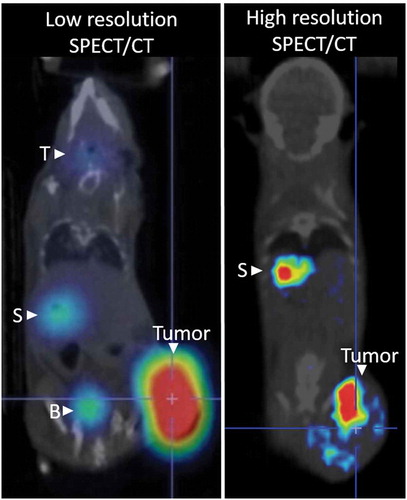
Table 3. Resolution and sensitivity of imaging instruments used to detect oncolytic virus replication via NIS mediated radiotracer uptake.