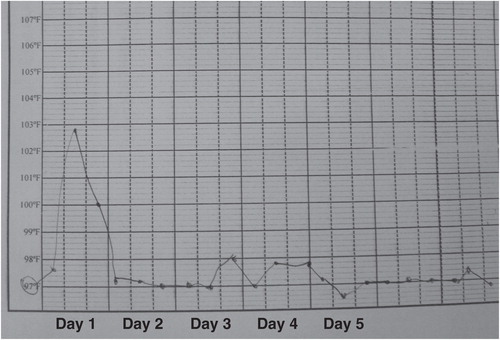
Figures & data
Table 1.
Summary of currently available monotherapies for VL*.
Table 2.
TPP for new clinical entities against VL (as monotherapy)*.
Table 3.
Potential regimens of LAMB that have been developed for use against VL*.
Box 1.
Review of LAMB treatment for cutaneous, mucosal and post-kala azar leishmaniasis as leishmaniasis also refer back to cutaneous and mucosal.
Table 4.
Summary of studies and clinical trials on LAMB use for VL.
Table 5.
Injectable liposomal products approved by the US FDA.
Table 6.
Overview of LAMB formulations introduced in the market or under development*.