Figures & data
Table 1. Currently approved targeted radionuclide therapies in oncology.
Table 2. Selection of radionuclides of interest for targeted radionuclide therapy.
Figure 1. Antibodies and their derived antigen-binding fragments. A. Camelid heavy-chain-only antibody (HCAb) and its VHH (also known as nanobody or sdAb), bivalent and circulation-lifetime extended nanobody constructs. B. Conventional mAb and the derived Fab, scFv, Fv domains VL or VH, Fab’2, minibody and diabody.
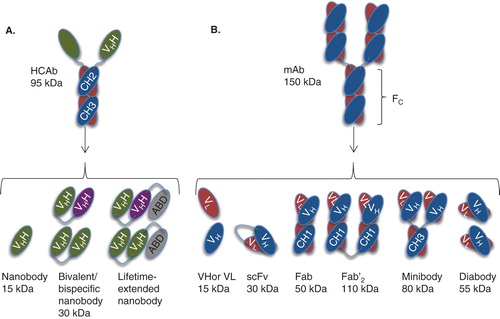
Table 3. Completed clinical trials of mAb-based targeted radionuclide therapies in oncology.
Figure 2. Specific diagnostic tumor imaging with nanobodies at 1 h post-injection. A. SPECT/CT images of mice injected with 99mTc-labeled anti-HER2 nanobody. B. PET/CT images of rats injected with 68Ga-labeled anti-HER2 nanobody. Animals on left carry HER2-positive-xenografted tumors, animals on right carry HER2-negative-xenografted tumors.
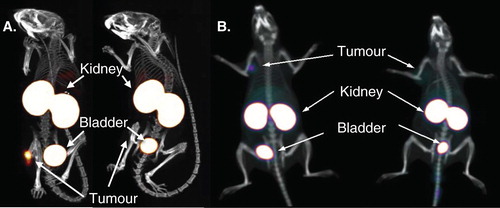
Figure 3. TRNT using a 177Lu-labeled anti-HER2 nanobody in HER2+ SKOV3 tumor-xenografted mice (subcutaneous, right hind leg, 20 – 30 mm3 at start of TRNT); A. Tumor growth monitoring during TRNT. Tumor volumes were quantified using (A.1.) caliper measurements (mm3) and (A.2.) bioluminescence imaging (ph/s/cm2/sr) as a function of time (days). Control groups (n = 8 per group) received either PBS or 177Lu-labeled non-targeting nanobody BCII10 (19.3 ± 0.8 MBq). Animals in the treatment group (n = 8) received a weekly i.v. injection of untagged 177Lu-labeled anti-HER2 nanobody 2Rs15d (20.7 ± 0.4 MBq). All treatments occurred with a 150 mg/kg Gelofusin co-injection. In terms of tumor growth, important differences were observed between the control groups and the treated group, for both caliper and bioluminescence measurements. B. Renal histopathology of 177Lu-dosed animal groups was compared to the PBS-treated animal group, 3 months after TRNT initiation. Sections were H&E stained and examined for signs of renal toxicity. No differences in renal histology were observed between the animal groups that received (B.1.) PBS, (B.2.) 177Lu-labeled BCII10 and (B.3.) 177Lu-labeled 2Rs15d.
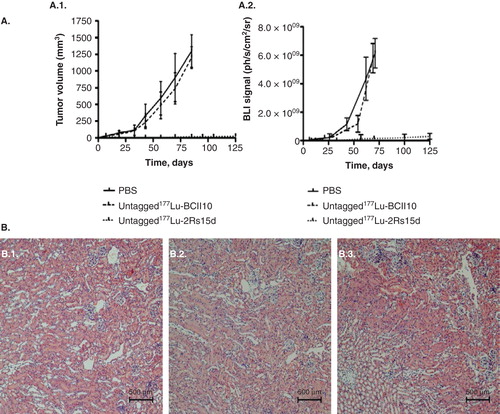
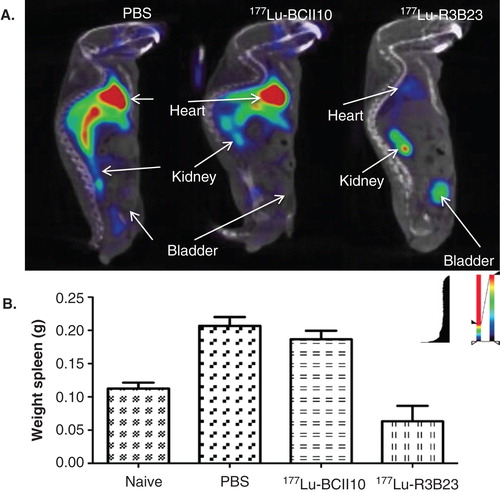
Figure 4. Prophylactic TRNT using 177Lu-labeled nanobody R3B23, which targets 5T2 multiple myeloma (MM) cell-produced M-protein. Syngeneic mice were i.v. injected with 2 × 106 5T2MM cells and TRNT started 1 week after tumor cell inoculation. These 5T2MM mice received a weekly i.v. injection of either PBS, 18.5 MBq 177Lu-labeled non-targeting nanobody BcII10 or 18.5 MBq 177Lu-labeled R3B23. A. Sagittal SPECT/micro-CT images 1 h after i.v. injection of 99mTc-R3B23 Nanobody in mice receiving TRNT for 5 weeks. 5T2MM mice that received 177Lu-R3B23 TRNT showed lower levels of circulating M protein that was captured by the 99mTc-R3B23 radiotracer than in control groups, a sign of delayed disease progression. B. Weights of spleens (homing site of 5T2MM tumor cells) after 7 weeks of TRNT with 177Lu-R3B23 or controls.